Question
Question: The qualitative sketches I, II and III given below show the variation of surface tension with molar ...
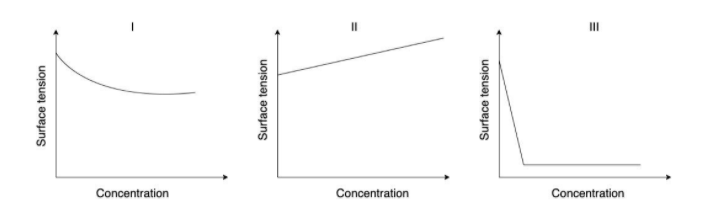
The qualitative sketches I, II and III given below show the variation of surface tension with molar concentration of three different aqueous solutions of KCl,CH3OH and CH3(CH2)11OSO3−Na+ at room temperature.
The correct assignment of the sketches is:
A. I:KCl;II:CH3OH;III:CH3(CH2)11OSO3−Na+
B. I:CH3(CH2)11OSO3−Na+;II:CH3OH;III:KCl
C. I:KCl;II:CH3(CH2)11OSO3−Na+;III:CH3OH
D. I:CH3OH;II:KCl;III:CH3(CH2)11OSO3−Na+
Solution
The surface tension of a liquid is linked with the magnitude of their intermolecular forces present in the liquid.
Complete step by step solution:
(I) In the figure we can see that the surface tension of aqueous solution is decreasing as the concentration increases.
When CH3OH is mixed with H2O to form an aqueous solution of CH3OH, the hydrogen bond between the water molecules present is broken and there is a decrease in the force of attraction. So when there is a decrease in the force of attraction between the molecules the surface tension will also decrease. That is why the aqueous solution of CH3OH decreases its surface tension when concentration is increased.
(II) In the figure we can see that the surface tension of aqueous solution is increasing when the concentration is increasing.
When we add KCl to water there is a heat of hydration. That means K+ and Cl− get solvated by water molecules. When this happens, there is an increase in the electrostatic force of attraction. So, when there is an increase in the force of attraction the surface tension also increases. That Is why the aqueous solution of KCl increases its surface tension when the concentration is increased.
(III) In the figure we can see that there is a sharp decrease in surface tension when concentration is increased.
When we add CH3(CH2)11OSO3−Na+ to water the, the surfactant molecules adsorb at the water surface. Now, at the surface some of the water molecules are replaced by the surfactants. The force of attraction between water and surfactants are lesser than the two water molecules. Therefore after some time, we can see a rapid decrease in the surface tension of the solution
Note:
1. For solving this easier, we could have started with surfactant and matching it with figure (III), as we know that it is a characteristic property of surfactants. And then we can move to KCl and finally to CH3OH. This way we could save a lot of time.
2. The surface tension of any pure fluids does not change with the concentration. It will remain the same whatever the concentrations are.
