Question
Question: The proportion of sigma, and pi bond in 1,4 – benzoquinone? A. \[1:3\] B. \[3:2\] C. \[2:1\]...
The proportion of sigma, and pi bond in 1,4 – benzoquinone?
A. 1:3
B. 3:2
C. 2:1
D. 2:3
Solution
To solve this question, we need to first understand the meaning of both sigma and pi bonds. Then, we need to draw the molecular structure of 1,4 – benzoquinone to identify the number of sigma and pi bonds.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Covalent bonds are formed when there is a sharing of electrons between two combining atoms. This sharing of electrons improves the stability of both the combining atoms. Both sigma bonds and pi bonds are formed in covalent compounds. Let us discuss them one by one:
1.Sigma bonds: Sigma bonds are formed by the direct overlapping atomic orbitals. This overlapping happens on the axial planes of both the combining atomic orbitals. Such a heads on overlapping takes place between s and s orbitals, and p and s orbitals. Sigma bonds are the strongest form of covalent bonds. These bonds are also commonly referred to as single bonds.
2.Pi bonds: Pi bonds are formed due to sidewise overlap of atomic orbitals. This bonding takes place over a direction which is perpendicular to the internuclear axis. When pi bonds are being formed, the axes of the atomic orbitals are parallel to each other. Pi bonds are weaker than sigma bonds. Pi bonds are also commonly referred to as double or triple bonds.
The molecular structure of 1,4 – benzoquinone can be given as:
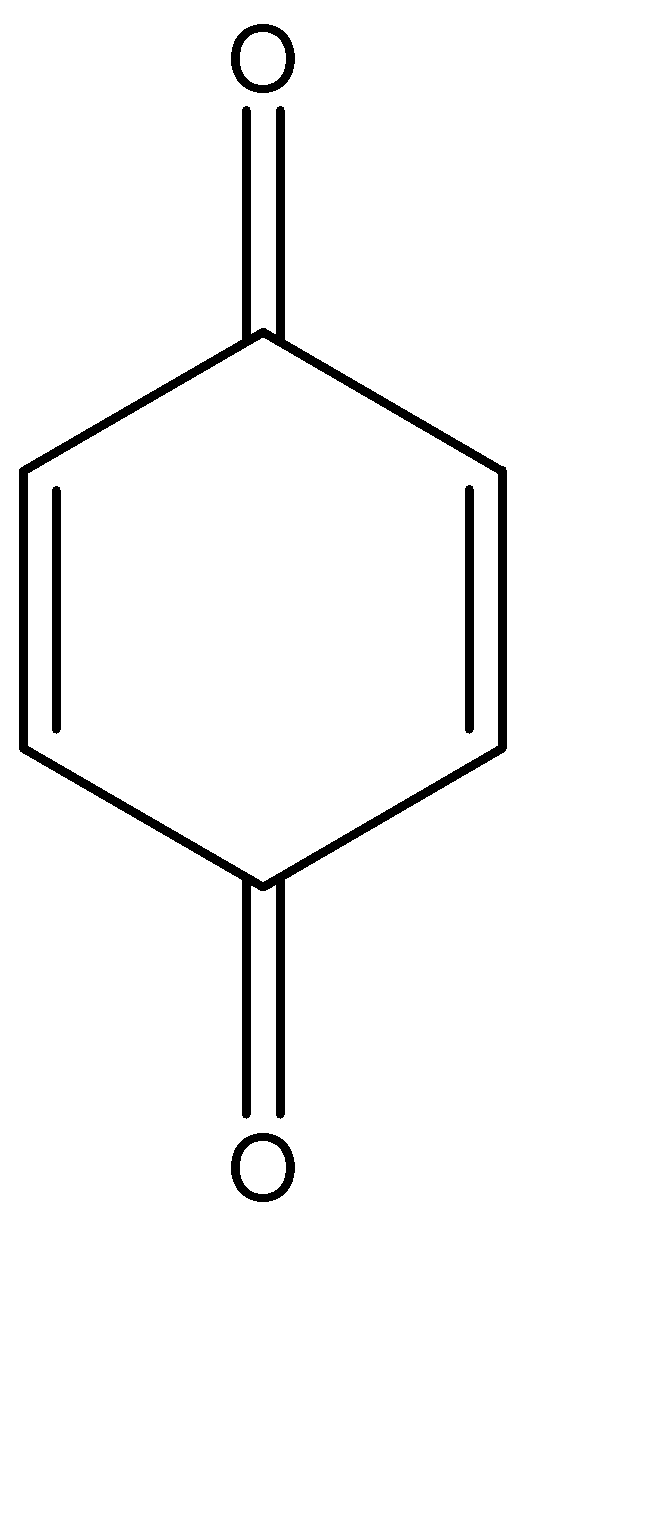
We can see that there are:
a)6 carbon – carbon single bonds
b)2 carbon – oxygen single bonds
c)2 carbon – carbon double bonds
d)2 carbon – oxygen double bonds
Hence, there are 8 single bonds and 4 double bonds present, there are 8 sigma bonds and 4 pi bonds present and the proportion of sigma, and pi bond in 1,4 – benzoquinone is 2:1
Hence, Option C is the correct option.
Note: Double bonds consist of one sigma and one pi bond, whereas a typical triple bond is made up of two pi bonds and one sigma bond. It is important to note that a combination of sigma and pi bonds is always stronger than a single sigma bond.
