Question
Question: The progress of reaction \( {{A}_{\left( g \right)}}\rightleftharpoons x{{B}_{\left( g \right)}}+y{{...
The progress of reaction A(g)⇌xB(g)+yC(g) with time is presented in figure. What is the value of Kc0 at 300K ?
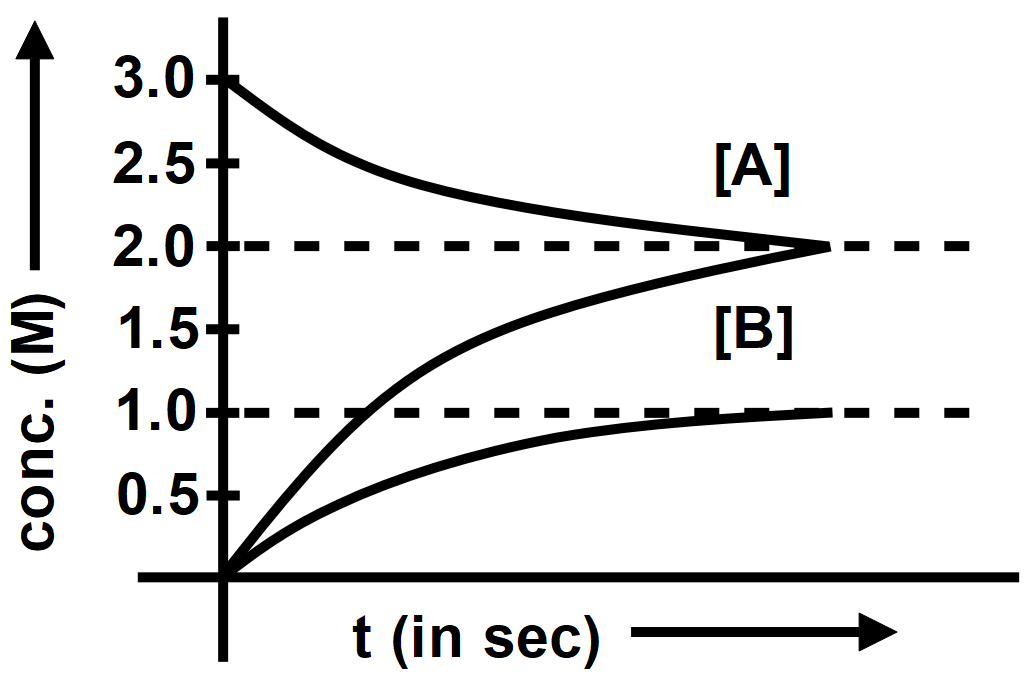
(A) 1
(B) 2
(C) 3
(D) 4
Solution
We know that the concentration of the product is zero. Still, with time, the concentration of the product increases and the concentration of the reactant decreases as it is getting consumed. After some time, the concentration does not change any further.
Complete answer:
As we know the equilibrium concentration equation, you need to know the formula for equilibrium constant Kc. When the chemical is in equilibrium, the ratio of the products to the reactants is called the equilibrium constant. We say that equilibrium has been reached when the reverse and forward reactions are proceeding at the same rate. For different reactions, those rates will become equal at various places in the transformation of reactant into a product. Therefore, it is not necessary for the equilibrium concentration of reactants and products to be the same. Changes in the concentrations of chemicals will shift chemical equilibrium according to Le Chatelier’s Principle.
When the concentration of a reactant is increased, the chemical equilibrium will shift towards the products. When the concentration of a product increases, the chemical equilibrium will shift towards the reactants. The concentration of A and B are the same and the same decrease in concentration of 2 M and the graphs show that condition so the value of equilibrium constant is 2.
Therefore, the correct answer is option B.
Note:
Remember that the temperature increases, molecules gain energy and move faster and faster. Therefore, the greater the temperature, the higher the probability that molecules will be moving with the necessary activation energy for a reaction to occur upon collision.
