Question
Question: The products (C) and (D) are :  and (D) are :
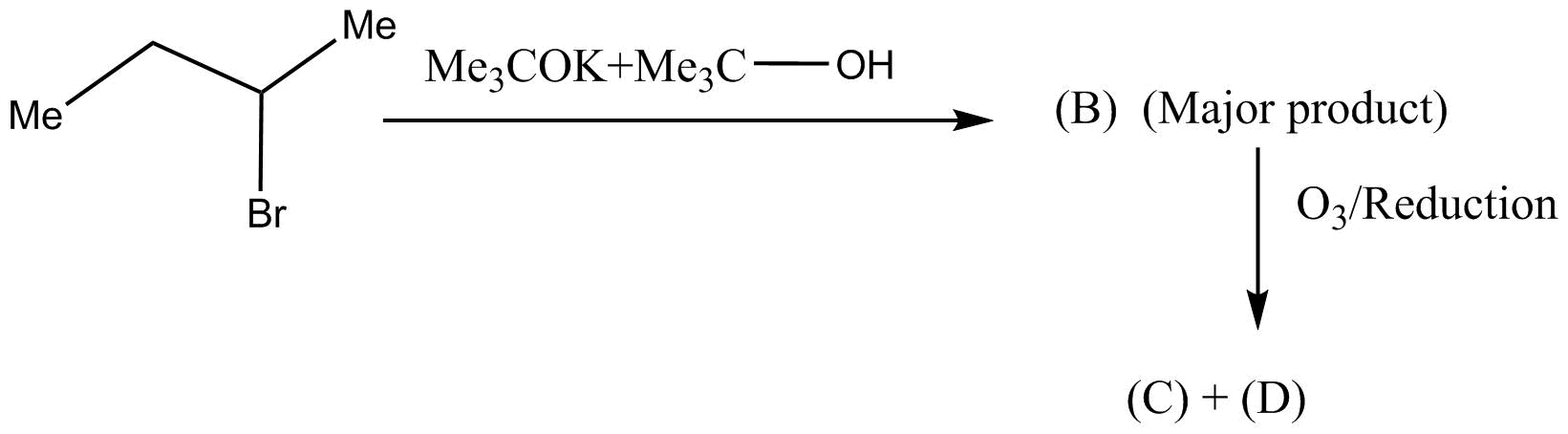
A.Methanol + propanal
B.Propanoic acid + CO2
C.2mol ethanoic acid
D.2mol ethanol
Solution
In the given question firstly we have analyzed the step by step process as in the first step of the reaction the breakage of each mole into two mole of product will take place. Now this two mole of majorly found product is then oxidised in order to make carboxylic acid in the ultimate and final process of reaction.
Complete step by step answer:
For the given reaction we have to find the step by step analysis of the process that is taking place in the given reaction.
Step 1: In the first step, the alkyl bromide is reacted with the given reactant inorder to form the product B. Now the alkyl bromide is dehydrobromination with the reaction of the sodium ethoxide in ethanol.
This given reaction is an accurate example of Saytzeff elimination. In the process of Saytzeff elimination more substituted alkene is formed.
Step 2: In the next step of the reaction, the reaction is of the nature of oxidative ozonolysis. This happens in order to form the dual molecule of the ethanoic acid. The C=C double bond will break and therefore the C atoms attached to C=C double bond are thus oxidized in the process.
This makes the correct option as C, 2mol ethanoic acid.
Note:
The founder of the rule which governs the reaction is Russian chemist Alexander Zaitsev, University of Kazan. He also studied a variety of different elimination reactions and observed a general trend in the resulting alkenes. Based on this trend he observed he stated that, "The alkene formed in greatest amount is the one that corresponds to removal of the hydrogen from the alpha-carbon having the fewest hydrogen substituents."
