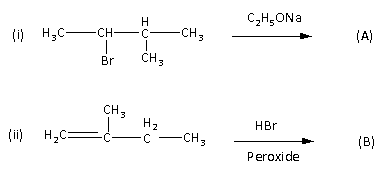
Question
Question: The products (A) and (B) are respectively  and (B) are respectively
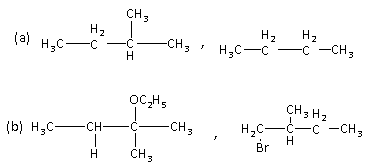
Solution
In the first reaction alkyl halide in presence of the base undergoes the nucleophilic substitution reaction. In the second reaction, unsaturated alkene reacts with hydrogen bromide in presence of peroxide leads to give the product as per the anti-Markovnikov rule.
Complete solution:
Alkyl halide on reaction with a base like sodium ethoxide as given in reaction number (i) shows nucleophilic substitution reaction.
The mechanism of the reaction is as follows:
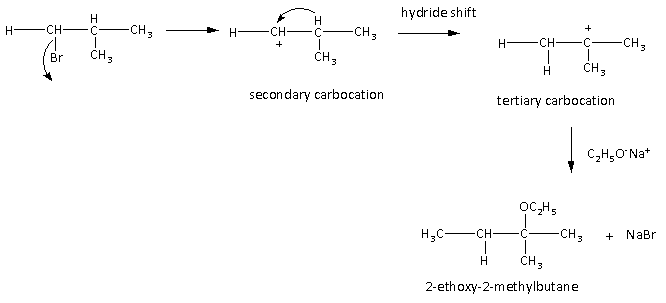
Here, we can see as bromide removed carbocation is formed which is secondary carbocation which is undergoing hydride shift and forms tertiary carbocation. Then the nucleophile that is ethoxide ion attacks at the carbocation and gives the product that is 2-ethoxy-2-methyl butane.
In the case of the second reaction alkene in presence of hydrogen bromide shows that is the anti-Markovnikov rule.
According to the anti-Markovnikov rule, the negative part of the reagent is bonded to the less substituted carbon atom.Here, the alkene is 2-methylbut-2-ene that is asymmetric to different products.
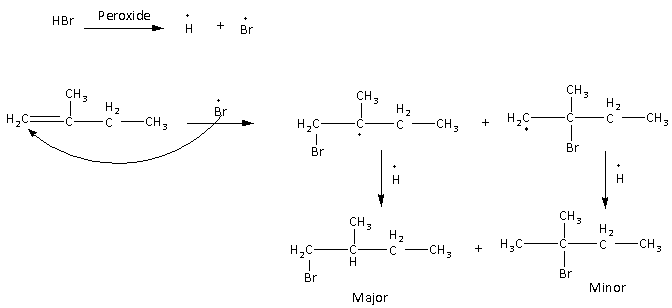
Here, we can see that in the first step of the reaction the radicals are formed. The formed radical then reacts with the alkene leads to form two intermediates which then results in two different products. Among them, the less substituted product is major as per the anti-Markovnikov rule.
Here, the major product of the reaction is 1-bromo-2-methyl butane.
Hence, the option (B) is the correct answer to the question.
Note: Reaction of an alkyl halide with base is either nucleophilic substitution or elimination where halide ion is removed from the alkyl halide. Among the hydrogen halides, only hydrogen bromide shows the peroxide effect. The peroxide effect is also known as the anti-Markovnikov effect.
