Question
Question: The product ‘X’ in the given reaction sequence is: \( C{H_3}COOC{H_3} + PhMgBr\;({\text{excess)}}...
The product ‘X’ in the given reaction sequence is:
CH3COOCH3+PhMgBr(excess)→ProductH+X
A. 1,1-diphenyl ethanol
B. 1,1-diphenyl methanol
C. methylphenyl ethanol
D. methyl phenyl ketone
Solution
A Grignard reagent is a chemical compound with general formula R−Mg−X , where X is a halogen and R is any alkyl or aryl group. It is one of the popular organometallic reagents which is used in organic reactions for creating new carbon-carbon single bonds.
Complete answer:
Two most common examples of Grignard reagent are phenylmagnesium bromide and methylmagnesium chloride. When methyl acetate reacts with excess of phenylmagnesium bromide in acidic medium, formation of a tertiary alcohol takes place. The reaction mechanism involved in the process is discussed below:
Step-1: Nucleophilic attack of phenyl group takes place at the carbonyl centre and an intermediate product is formed. The reaction proceeds as follows:
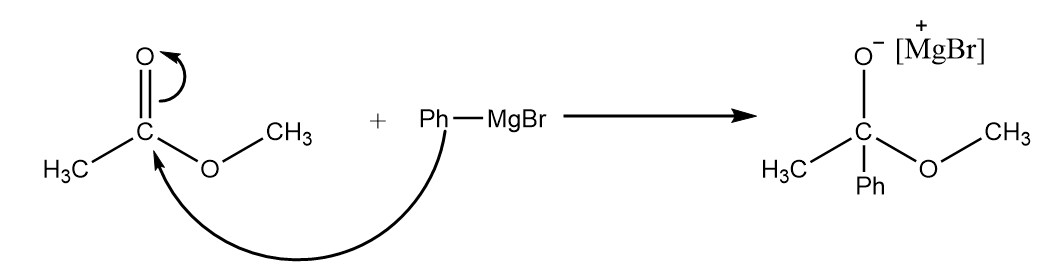
Step-2: When two oxygen atoms are present on a carbon atom, then one of the oxygen atoms has the tendency to form a double bond with the carbon atom, so the formation of ketone will take place by removing the leaving group. The reaction takes place as follows:
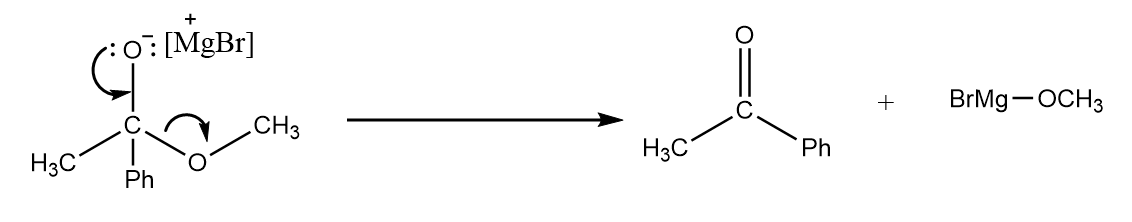
Step-3: As the Grignard reagent is present in excess, it again attacks the carbonyl centre of the compound and the formation of an intermediate compound takes place. The reaction proceeds as follows:
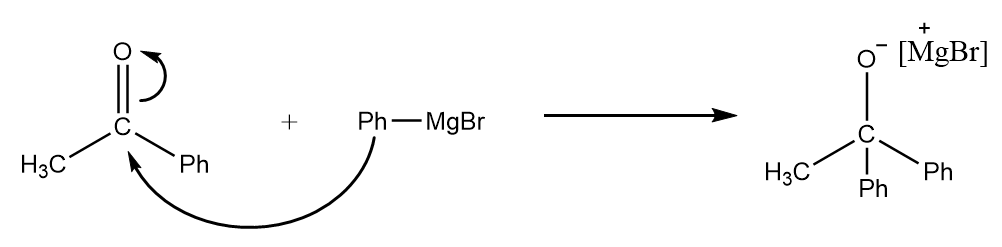
Step-4: The intermediate formed in the previous step will undergo protonation in acidic medium and formation of tertiary alcohol takes place. The reaction takes place as follows:
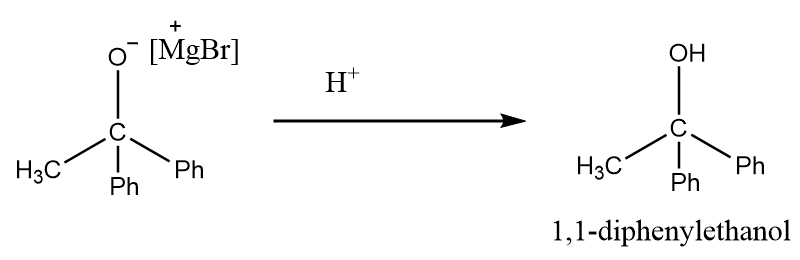
Hence, in the given reaction sequence, the product ‘X’ formed is 1,1-diphenyl ethanol.
Thus, option (A) is the correct answer.
Note:
It is important to note that the Grignard reagent does not react with the protected carbonyl group i.e., acetal and hemiacetal compounds because these compounds are base proof. Except these, the Grignard reagent is most commonly used for alkylation of carbonyl compounds.
