Question
Question: The product X and Y are respectively: 
A.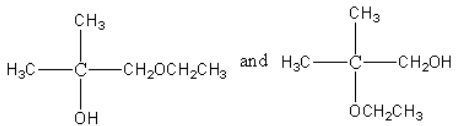
B.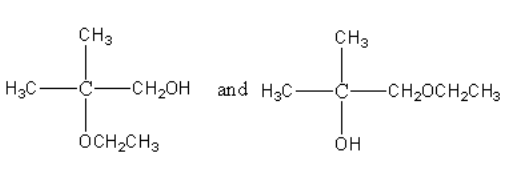
C.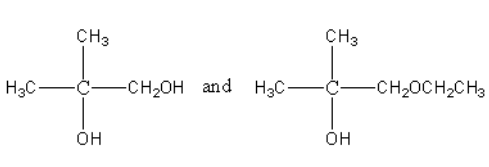
D.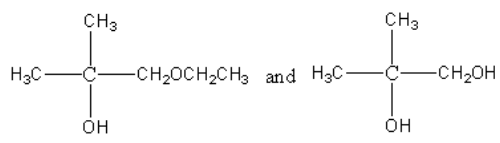
Solution
The given reaction is the ring opening reaction of an asymmetric epoxide. Depending on reaction condition asymmetric epoxide undergoes ring opening reaction by SN1 or SN2 mechanism. In the basic medium ring, the opening takes place by SN2 mechanism while in the acidic medium ring opening take place by SN1 mechanism.
Complete step-by-step answer:
The reaction given to us is :
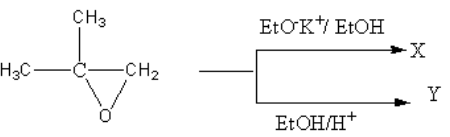
Here we have to identify products X and Y.
The reagent given for product X is EtOK + /EtOH . It is a basic reagent so the ring opening reaction will be carried out by SN2 mechanism. In SN2 mechanism, the nucleophile prefers to attack a less hindering side that is less substituted carbon atom.
Hence, the nucleophile EtO− will attack on CH2and product X will be as follows:
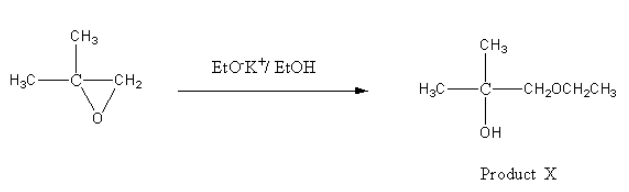
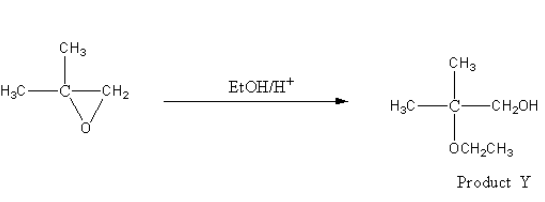
The reagent given for product Y is EtOH/H + . It is an acidic reagent so the ring opening reaction will be carried out by SN1 mechanism. In SN1 mechanism, the nucleophile prefers to attack more substituted carbon atoms.
Hence, the nucleophile EtO− will attack on C(CH3)2 carbon and product Y will be as follows:
Thus, product X and Y are
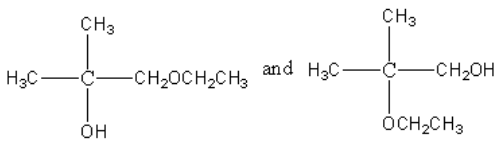
Hence, the correct option is (A).
Note: Asymmetric epoxide shows regioselectivity of product. Symmetric epoxides can give only one product however, asymmetric epoxides give two possible products. Using the reaction condition we can determine the major product out of two possible products. In a basic medium, more substituted alcohol is the product while in an acidic medium less substituted alcohol is the product.
