Question
Question: The product which is not formed in the following reaction: ``` CH3 CH3 | ...
The product which is not formed in the following reaction:
CH3 CH3
| |
Ph-C-CH-CH-CH-CH2OH
| | |
OH OH OH
HIO4 (excess)
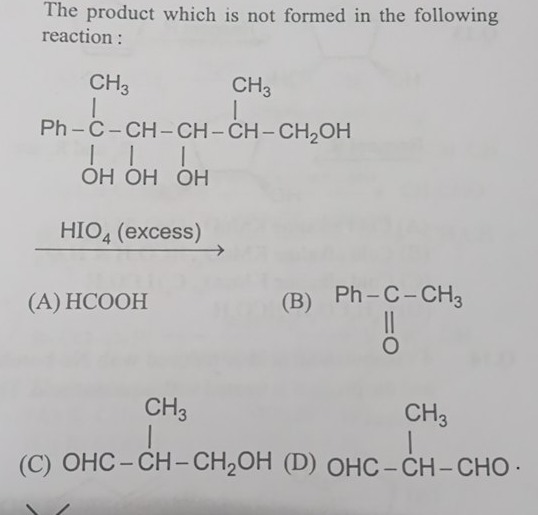
HCOOH
CH3
|
Ph-C-CH3
||
O
CH3
|
OHC-CH-CH2OH
CH3
|
OHC-CH-CHO.
C and D
Solution
The reaction involves the oxidation of a polyol with periodic acid (HIO4) in excess. Periodic acid cleaves carbon-carbon bonds between adjacent carbons bearing hydroxyl groups (vicinal diols).
The given substrate has hydroxyl groups on carbons C1,C2,C3,C4, and C5, where: C1: Ph-C(OH)(CH3)- C2: -CH(OH)- C3: -CH(OH)- C4: -CH(OH)- C5: -CH2OH
The vicinal diol cleavages occur at the bonds: C1−C2, C2−C3, C3−C4, and C4−C5.
Upon cleavage and oxidation:
- A tertiary alcohol (like C1) becomes a ketone. The fragment from C1 is Ph-C(=O)-CH3 (Acetophenone). This matches Option (B).
- A secondary alcohol (like C2,C3,C4) becomes an aldehyde (-CHO).
- A primary alcohol (like C5) becomes formic acid (HCOOH). This matches Option (A).
The CH3 group is attached to C1. Since C1 is oxidized to a ketone (Acetophenone), the CH3 group remains attached to this ketone.
Options (C) and (D) depict fragments containing a CH(CH3) unit where the CH is part of an aldehyde. This is not possible because the CH3 group is exclusively on C1, which forms acetophenone and does not become an aldehyde fragment. Therefore, products (C) and (D) are not formed.
