Question
Question: The product (P) in the following reaction can be represented as...
The product (P) in the following reaction can be represented as
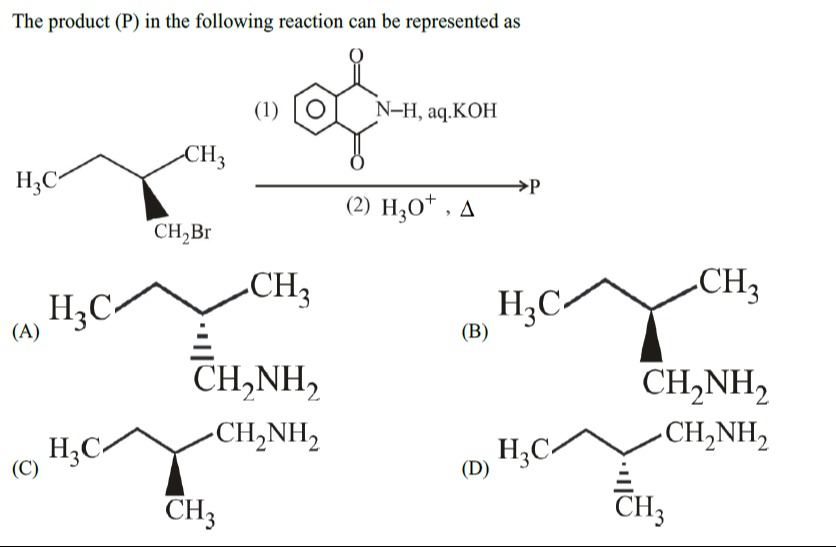
H3C CH3 CH2NH2
H3C CH3 CH2NH2
H3C CH2NH2 CH3
H3C CH2NH2 CH3
A
Solution
The reaction shown is a Gabriel synthesis, which converts an alkyl halide into a primary amine. The starting material is 1-bromo-2-methylbutane. The Gabriel synthesis involves nucleophilic attack by the phthalimide anion on the carbon bearing the bromine atom. In this case, the bromine is on a primary carbon (CH2Br), so the reaction proceeds via an SN2 mechanism. The carbon atom bearing the bromine is not chiral.
The chiral center in the molecule is at position 2, which is bonded to an ethyl group (CH2CH3), a methyl group (CH3), a hydrogen atom, and the bromomethyl group (CH2Br). The Gabriel synthesis replaces the -Br with -NH2 without affecting the stereochemistry at the chiral center. Therefore, the stereochemistry of the chiral center in the starting material is retained in the product.
Let's determine the stereochemistry of the starting material, (R)-1-bromo-2-methylbutane. The groups attached to the chiral carbon are:
- CH2Br (priority 1)
- CH2CH3 (priority 2)
- CH3 (priority 3)
- H (priority 4)
In the given representation, CH2Br is on a wedge, and CH3 is on a dash. Assuming the ethyl group is to the left and H is to the right:
- CH2Br (wedge)
- CH2CH3 (in plane, left)
- CH3 (dash)
- H (in plane, right)
To determine the configuration, we can swap the positions of the methyl group (dash) and the hydrogen atom (in plane) to place the lowest priority group (H) in the back. This swap inverts the configuration. New arrangement:
- CH2Br (wedge)
- CH2CH3 (in plane, left)
- H (dash)
- CH3 (in plane, right)
Tracing from priority 1 to 2 to 3 (CH2Br to CH2CH3 to CH3) is counter-clockwise. Since H is in the back (dash), this configuration is S. Because we performed one swap, the original configuration of the starting material was R.
The product is (R)-2-methylbutan-1-amine. Now let's check the options:
Option (A): H3C−CH2−CH(CH3)−CH2NH2 The chiral center has groups:
- CH2NH2 (priority 1)
- CH2CH3 (priority 2)
- CH3 (priority 3)
- H (priority 4)
In option (A), CH2NH2 is on a wedge, and CH3 is on a dash. Assuming ethyl is left and H is right:
- CH2NH2 (wedge)
- CH2CH3 (in plane, left)
- CH3 (dash)
- H (in plane, right)
Since CH3 is in the dash position, we can directly determine the configuration by tracing 1 -> 2 -> 3 (CH2NH2 to CH2CH3 to CH3). This rotation is clockwise. Therefore, the configuration is R. Option (A) matches the expected (R) configuration.
Options (B), (C), and (D) represent different stereoisomers or constitutional isomers that do not match the product of the reaction with the correct stereochemistry.
