Question
Question: The product of following reactant is: 
A.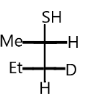
B. 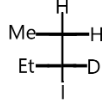
C.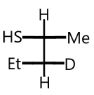
D. 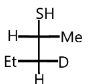
Solution
Potassium hydrosulphide is an inorganic compound. It is colorless salt. It is made up of cation K+ and bisulfide anion SH−. It will act as a nucleophile in this reaction. To find out the product of the above reactant first, we will identify whether it is primary or secondary or tertiary chiral carbon. After that we will see the role of KSH.
Complete step-by-step answer: As we know that the secondary and tertiary chiral carbon forms the product through SN2 mechanism. The reagent KSH acts as a nucleophile then it will displace the halogen in the compound. During SN2 mechanism no carbocation is formed but transition state is formed. They occur in one step. During the reaction nucleophile attacks from the back side. Since the nucleophile attacks from the back side, we get a product having configuration opposite to the reactant i.e. inversion of configuration takes place in SN2 reaction which is also said to be Walden inversion (complete inversion occurs).
According to the above conditions, let us see our problem. When the reactant reacts with potassium hydrosulfide, the halogen that is iodine is replaced by the SH− ion. Elimination of potassium iodide will take place. The potassium hydrosulphide will split into anion and cation as:
KSH→K++[SH]−
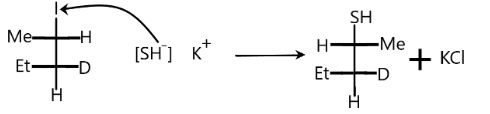
Nucleophile attacks from the back side, therefore we get products with opposite configurations to the reactant.
So, option (D) is correct.
Note: Keep in mind that only a strong nucleophile has the ability to displace the weaker nucleophile from the compound. Whenever any nucleophile attacks from the back side, the configuration of the product will be opposite to the reactant. Don’t confuse yourself with the electrophilic and nucleophilic.
