Question
Question: The product obtained in the reaction of \[C{H_3}CON{H_2}\] and \(NaN{O_2}/HCl\) is – (A).\(C{H_3}...
The product obtained in the reaction of CH3CONH2 and NaNO2/HCl is –
(A).CH3COOH
(B) CH3COCH3
(C) CH3CON⊕H3C(−)l
(D) CH3NH2
Solution
Acetamide is an organic compound which releases nitrogen gas from the solution when reacted with NaNO2/HCl.It is the simplest amide derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent.
Complete step by step answer:
We know the given compound CH3CONH2 commonly known as acetamide is a organic compound with structure as: -
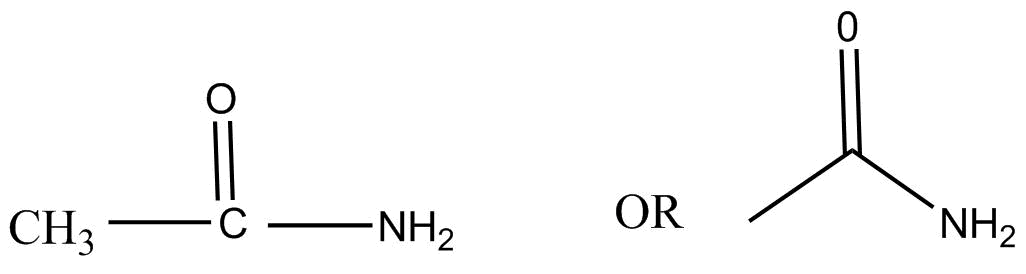
It is the simplest amide with IUPAC name ethanamide and is derived from acetic acid by the reaction with ammonia
CH3COOH(Acetic Acid) + H−NH2 (Ammonia) → CH3CONH2 (Ethanamide)
In industries, acetamide is produced by dehydrating ammonium acetate or via hydration of acetonitrile.
i.e. CH3CN+H2O→CH3CONH2
Ethanamide is used as a plasticizer and an industrial solvent. It has uses in electro chemistry and organic synthesis of pharmaceuticals, pesticides and antioxidants for plastics. When CH3CONH2 is reacted with NaNo2 in the presence of HCl, we get CH3COOH(acetic acid) as the main product.
Note:
Acetic acid will also be formed from ethanamide if the reagent is HNO2. Moreover, when ethanamide is treated with water, it also yields acetic acid.
i.e. CH3CoNH2+H2O→CH3COOH+H2O
