Question
Question: The product obtained in the following reaction is 
A. 2CH3CHO
B. 3HCHO
C. 3HCO2H
D. 3CH3OH
Solution
1,3,5− Trioxane is a white solid with a chloroform odor and is also known as trioxane or trioxin is a chemical compound with a molecular formula C3H6O3 . Trioxane is a stable cyclic trimer (a trimer is a molecule formed by the combination of three molecules of the same substance) of formaldehyde.
Complete answer:
In 1,3,5− Trioxane is a saturated organic heteromonocyclic compound and the molecular backbone contains a six-membered ring with three carbon atoms alternating with three oxygen atoms.
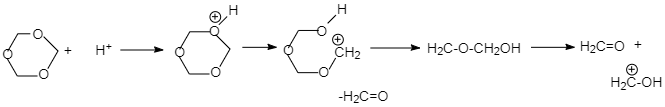
When a proton reaches the neighboring oxygen atom, it forms an OH bond of general length, along with an asynchronous modulation of the corresponding C-O bond lengths. This step is followed by ring opening and formation of carbocation which is unstable so it expels on a formaldehyde molecule to form another carbocation which then finally dissociates into one neutral and one protonated formaldehyde molecule.
1,3,5− Trioxane in the presence of Hydronium gives 3 formaldehyde.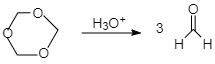
Therefore the correct answer is option B.
Additional Information: Trioxane can be acquired by the acid-catalyzed cyclic trimerization of formaldehyde in concentrated aqueous solution. Trioxane is mixed with hexamine and compressed into solid bars to make hexamine fuel tablets used as cooking fuel by the military.
Note:
Formaldehyde undergoes polymerization to form some of the important polymers like for example when formaldehyde is allowed to stand at room temperature, it slowly undergoes polymerization and forms a white solid called trioxane or metaformaldehyde.
Formaldehyde condenses with phenol in the presence of base and forms a cross-linked polymer called Bakelite.
