Question
Question: The product C is ? 
A.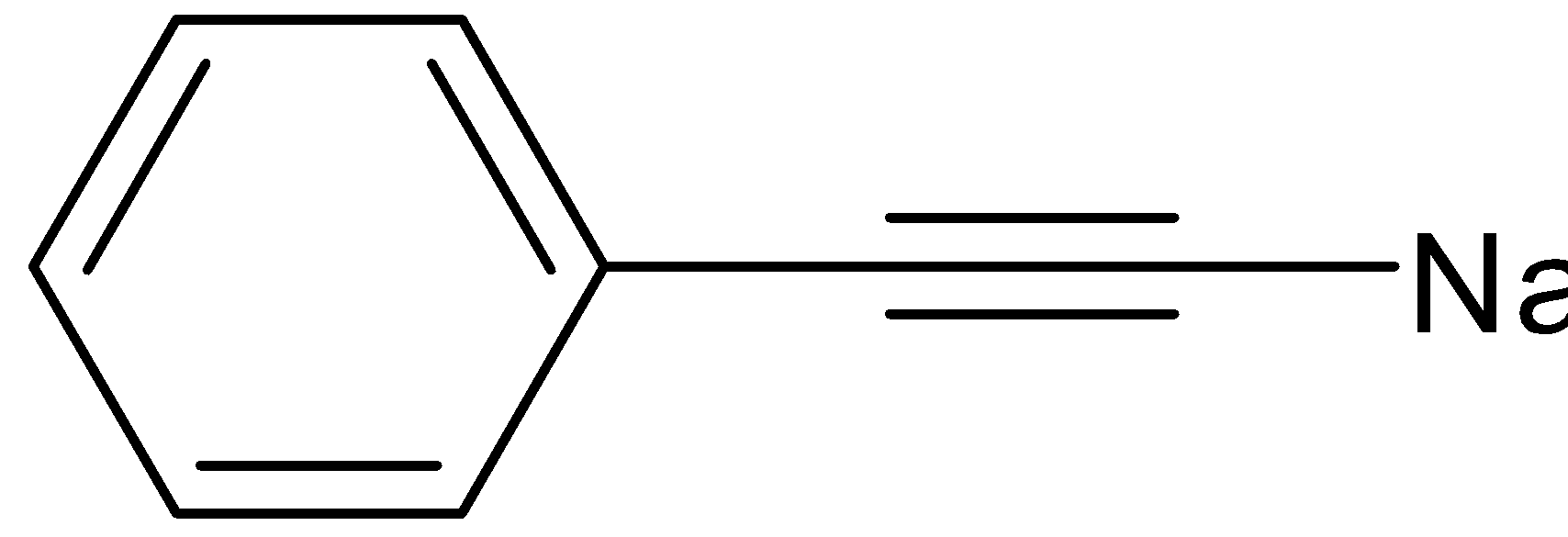
B.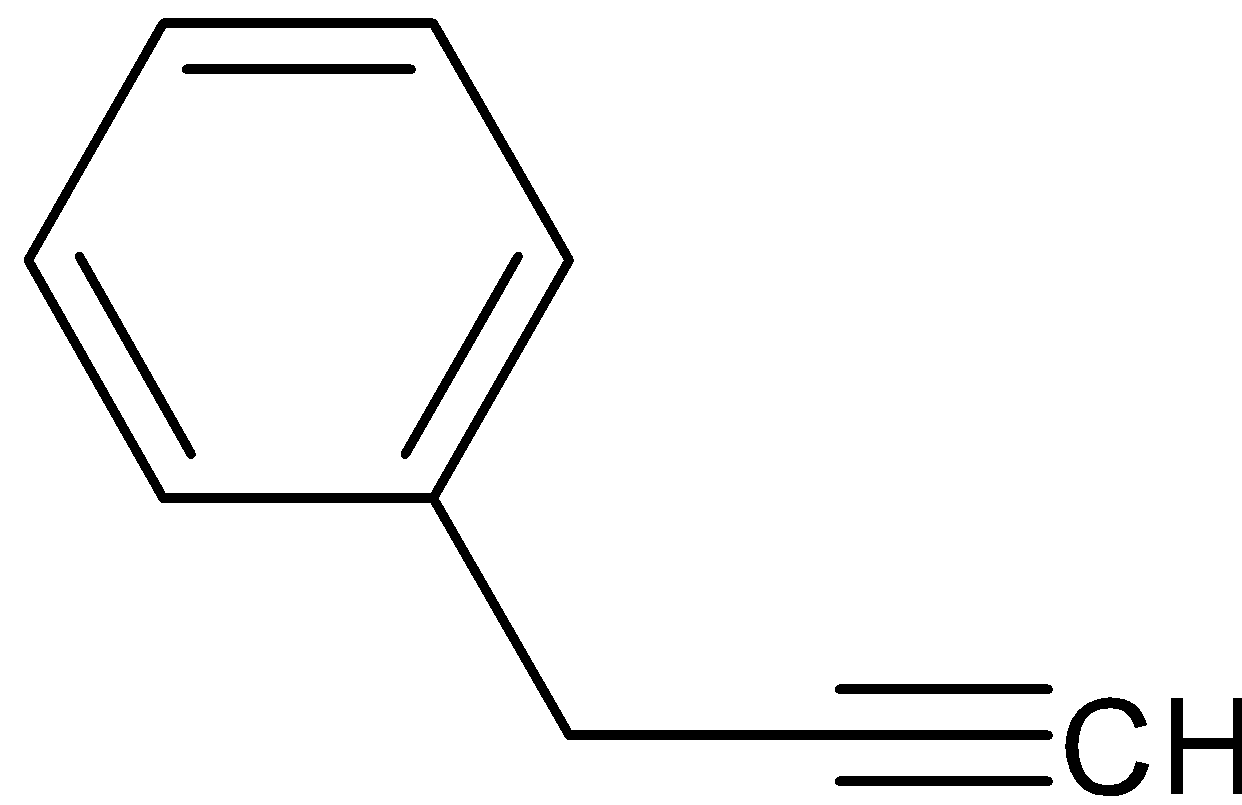
C.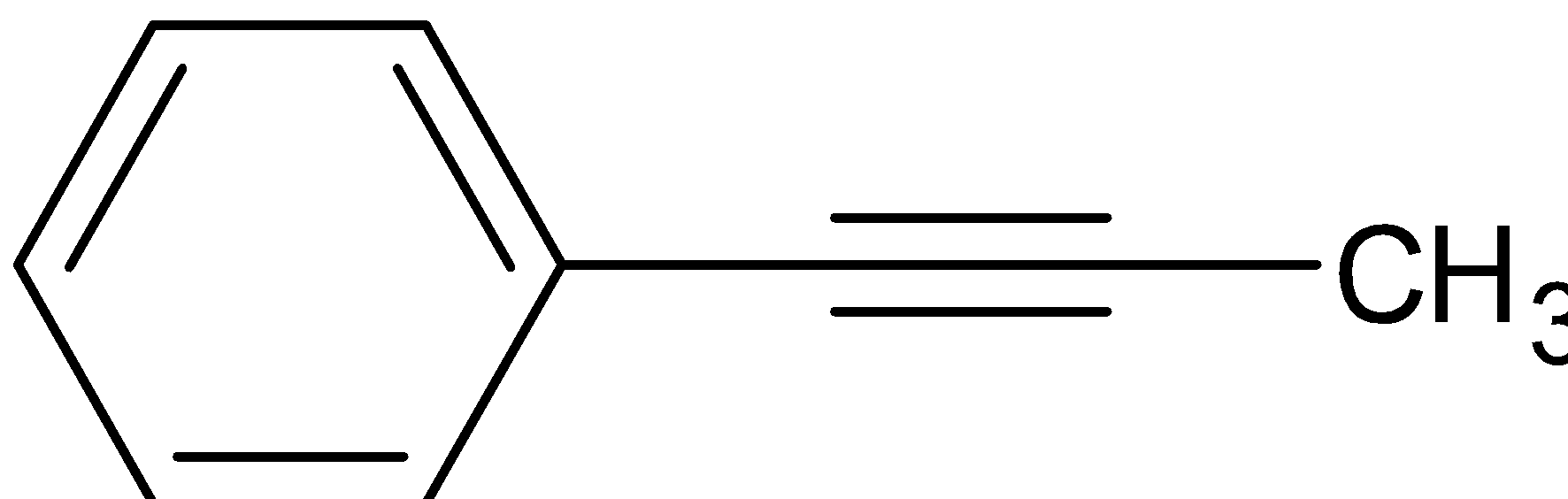
D.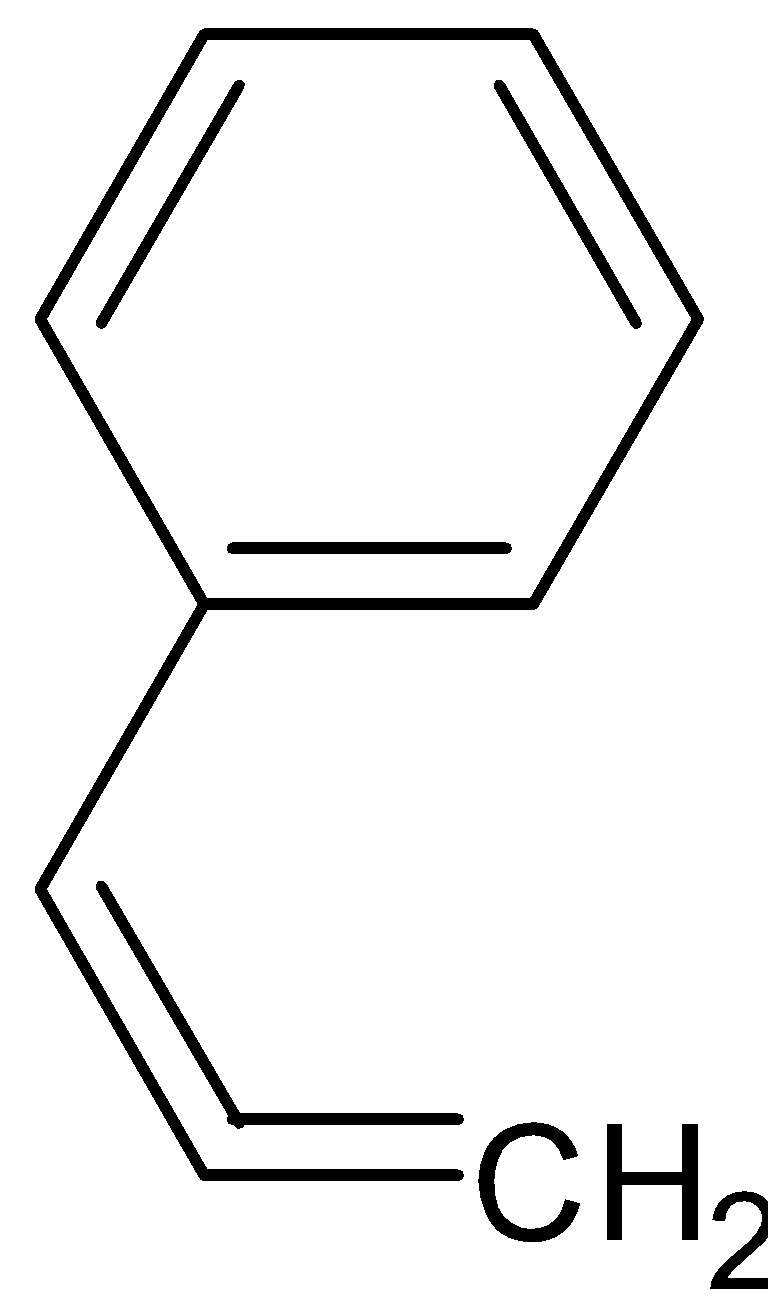
Solution
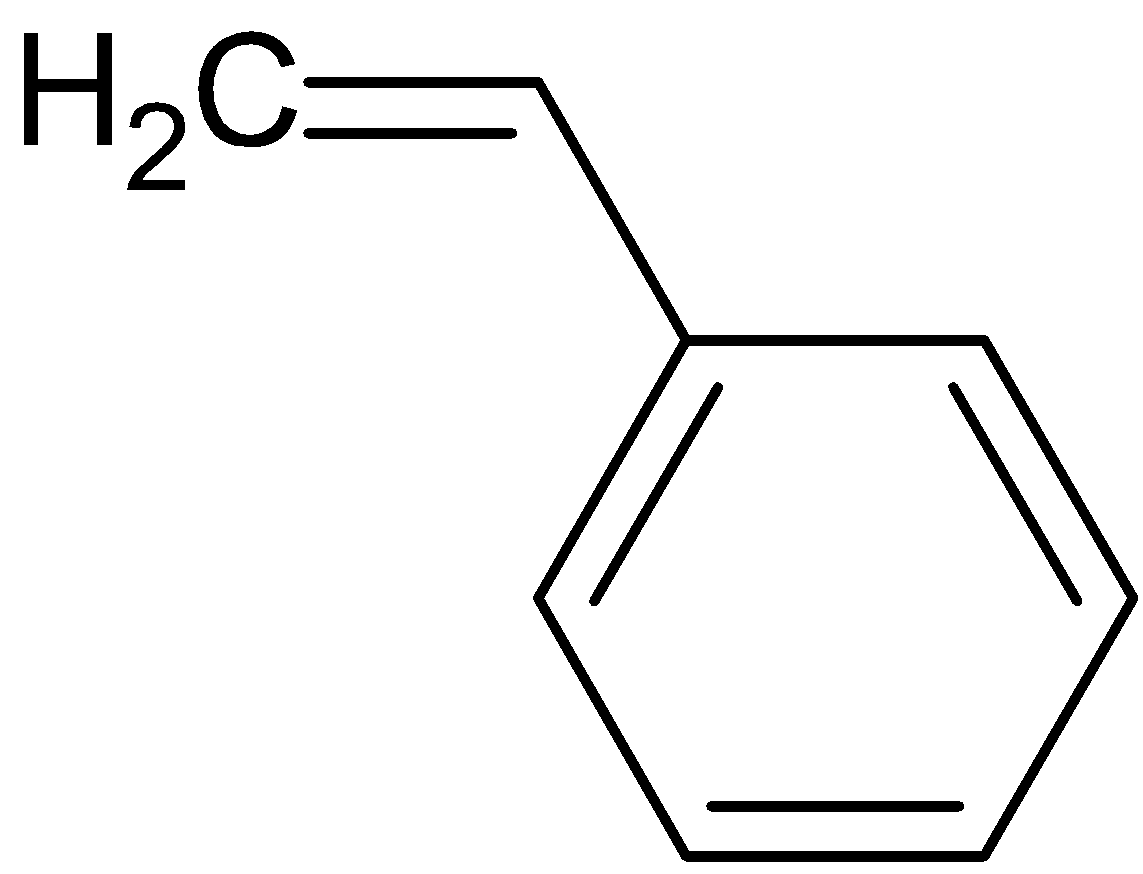
styrene is a liquid hydrocarbon which is highly important because of its marked tendency to undergo polymerization, polymerization is a process in which individual molecules are linked to produce extremely large molecules. Styrene is used in the manufacture of polystyrene which is an important plastic used widely.
Complete step by step solution:
Since styrene has alkene in its side chain it can undergo most of the reactions which are given by alkene. Firstly styrene undergoes bromination in presence of the solvent CCl4. So here the alkene side chain will undergo bromination reaction giving vicinal dibromides. Bromine s add to opposite faces of the double bond that is anti addition takes place here. Solvent CCl4 has no effect on the reaction. We can write the reaction as
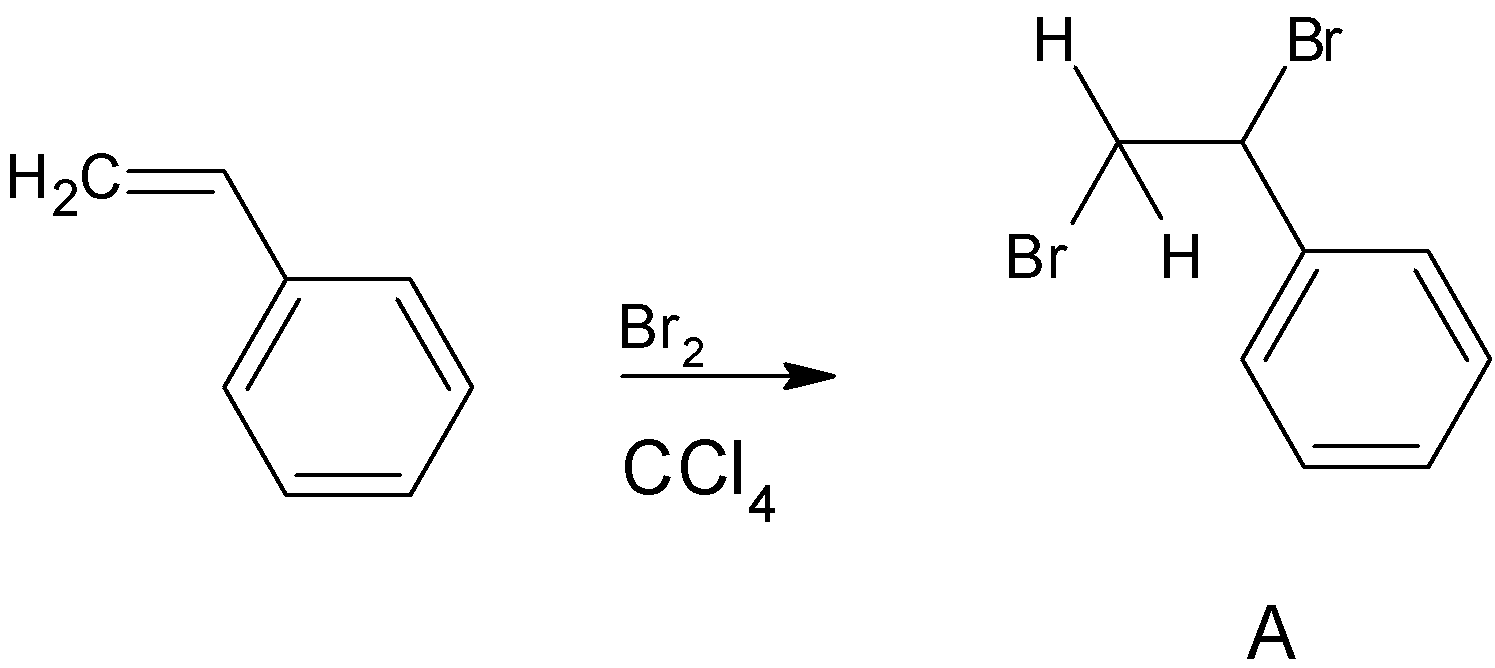
Next step the product A reacts with NaNH2 in presence of alcoholic KOH. Where NaNH2 is a strong base and excellent nucleophile which is used for deprotonation of weak acids and also for elimination reactions.one of the most common applications of NaNH2 is formation of alkynes from halogens. That is treatment of geminal dihalides and vicinal dihalides with NaNH2 gives corresponding alkynes.
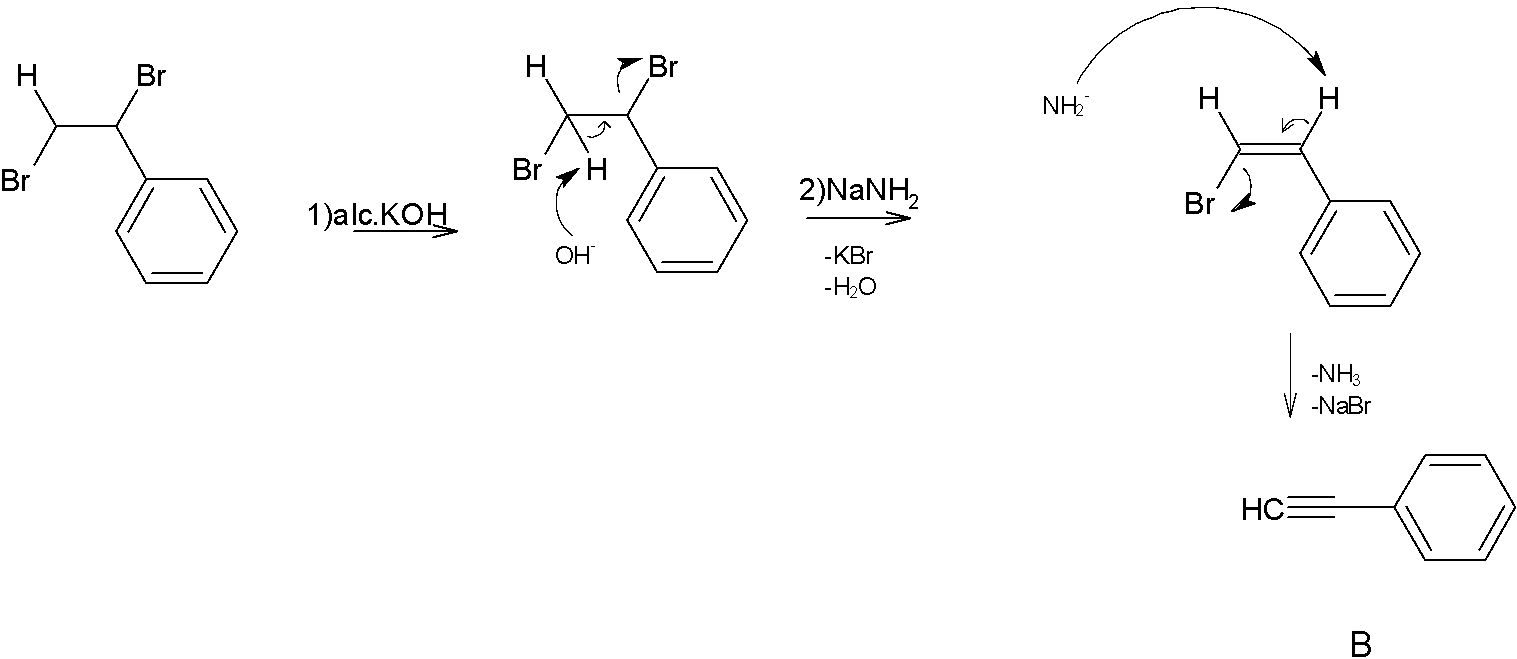
When product B is treated with NaNH2 and CH3Cl, The strong base NaNH2 will take the hydrogen atom from the terminal carbon atom to make it electron rich, which attracts the carbocation CH3+.
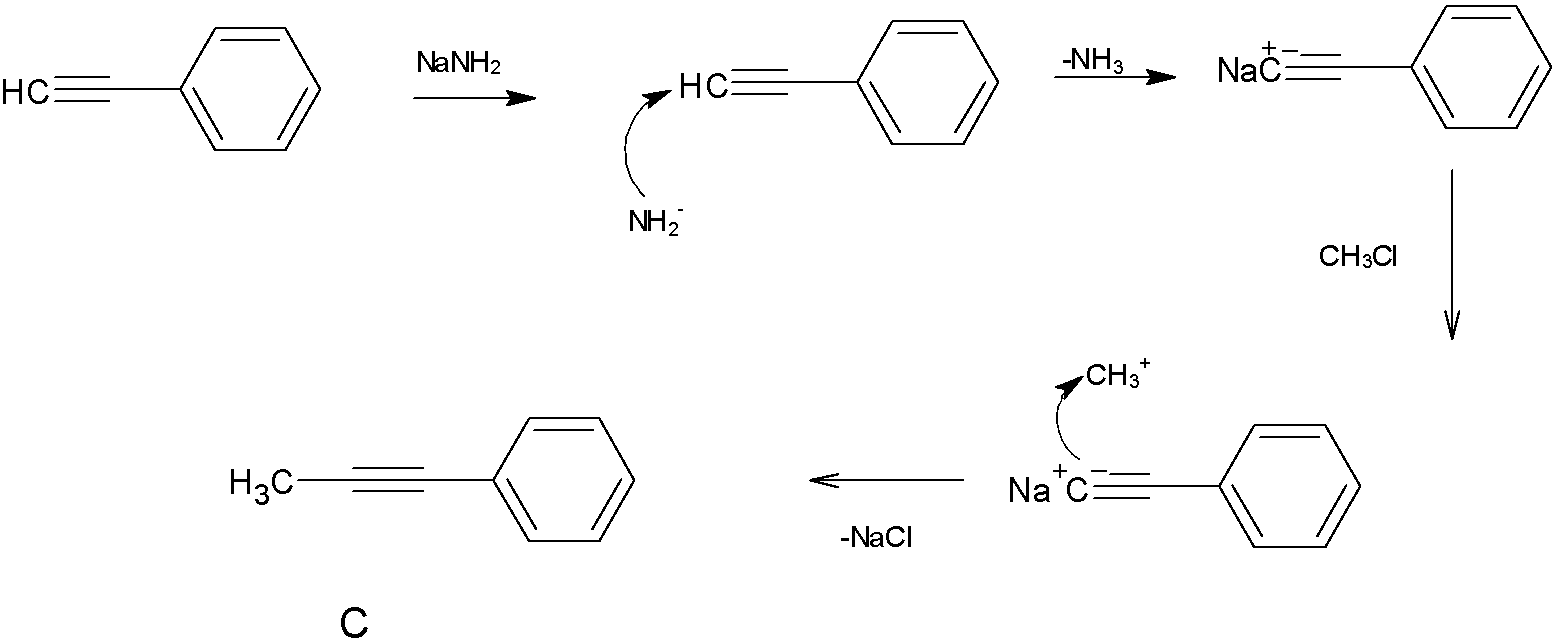
So here we get the product,its structure is given below
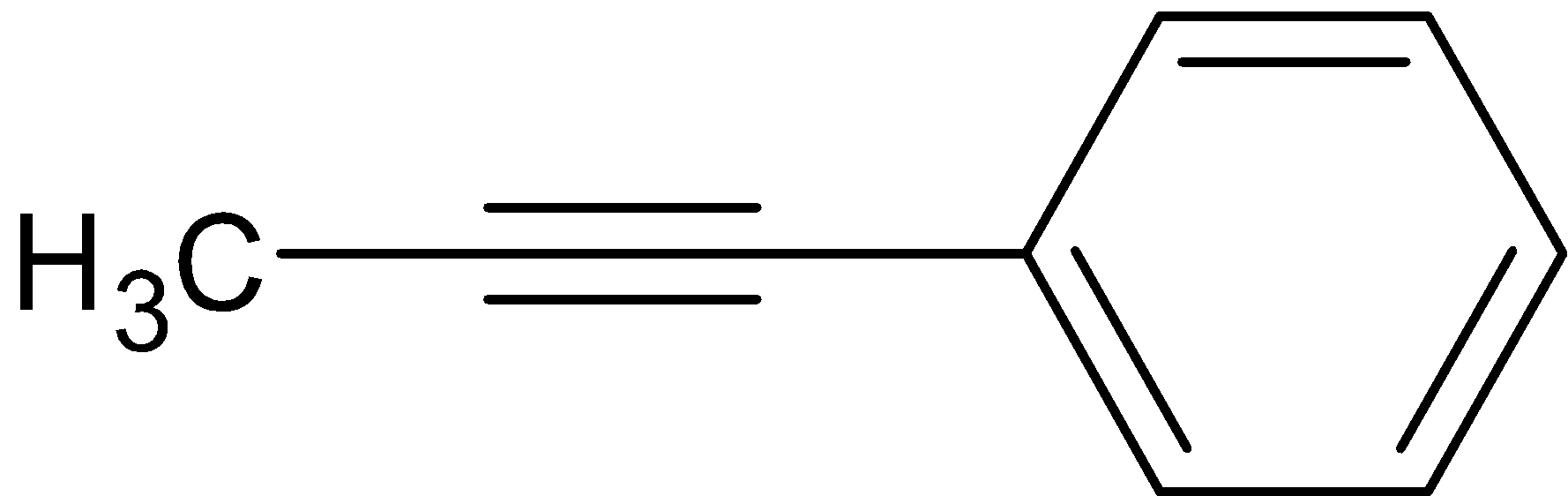
Hence, the correct answer is option C.
Note: Here the elimination reaction of di-bromides using NaNH2 to make alkenes require the presence of bases because it is E2 elimination. Formation of the product B is done by the elimination of H and bromine,which is an anti elimination that eliminates atoms are anti direction to each other, by which the product formed is an alkene with still a halide attached. This halide too can be removed to get the alkyne. Again the same E2 elimination mechanism is followed for this.
