Question
Question: The product B is? 
Solution
When the carbonyl compound reacts with hydroxyl amine then it forms oxime which is an organic compound belonging to the “imine” family having the general formula: RR’ C = NOH. The hydrolysis of the oximes in presence of the mineral acids results in the formation of the parent carbonyl compound from which it was formed.
Complete step-by-step answer: When cyclopropanone reacts with hydroxylamine, a condensation reaction takes place leading to the formation of the “ketoxime”. These oximes are colourless crystals that are soluble in water. in the course of the reaction, the nitrogen atom of the hydroxyl amine attacks the carbonyl carbon with the lone pair of electrons, this step is followed by the liberation of the water molecules as the condensation takes place and the oxime is formed as follows:
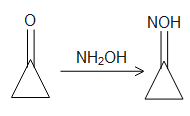
In the second phase of the reaction, the oxime undergoes hydrolysis when the acid molecule attacks the lone pair of the nitrogen atom. Thus the nitrogen atom becomes positively charged and thus abstracts the pi electron cloud from the carbonyl bond and thus leaves the carbonyl carbon positively charged. This carbon atom is then attacked by the nucleophilic water molecules and thus the oxime group leaves the carbonyl carbon and thus the ketone molecule is resumed again. Thus the product B is cyclopropane itself which is the starting compound. The reaction can be shown as follows:
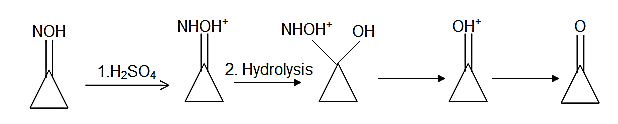
Note: The largest application or use of the oximes is as an intermediate in the industrial manufacture of “caprolactam”, a precursor of Nylon-6. About half of the world’s production of cyclohexanone is converted to the oxime and in the presence of sulphuric acid it undergoes rearrangement to form caprolactam.
