Question
Question: The Product (B) is:  is:
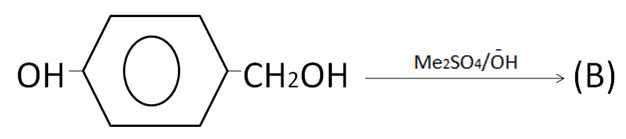
(A) 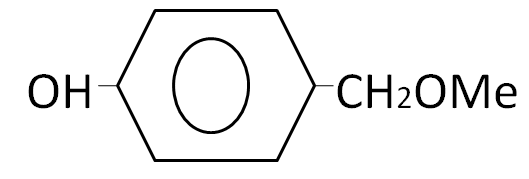
(B)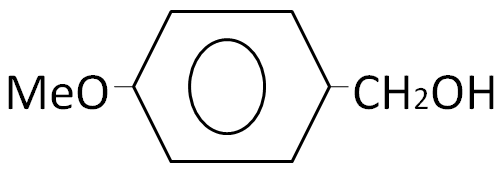
(C)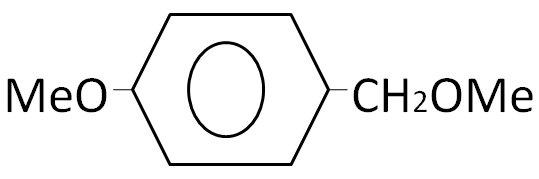
(D) all
Solution
Hint : Alcohols are those organic hydrocarbons which are attached with a hydroxyl group like methanol. Phenols are those organic hydrocarbons in which the benzene ring is in direct contact with the hydroxyl group. There are two types of nucleophilic substitution reactions namely substitution nucleophilic bimolecular reactions and substitution nucleophilic unimolecular reactions.
Complete Step By Step Answer:
Alcohol is the organic group in which carbon is in direct contact of the hydroxyl group like Methanol. Whereas the phenol is the organic group in which the benzene ring is in direct contact of the hydroxyl group like 1 Hydroxy benzene or commonly called the Phenol.
For the nucleophilic substitution reactions, we have two types of mechanisms namely substitution nucleophilic bimolecular reactions SN2 and substitution nucleophilic unimolecular reactions SN1 .
In SN2 the attack of the approaching group is from the back side of the alpha-carbon. Thus in the case of SN2 steric hindrance plays a vital role in the product formation. There is no reversible step in this mechanism, with the formation of the neutral transition state.
In SN1 the attack of the approaching group is from the front that is head on of the alpha-carbon. Thus the Steric hindrance does not affect the stereochemistry of the product on a large scale. The first step of the reaction is the formation of the carbocation as an intermediate which is reversible in nature and is the slowest step of the reaction. The formation of carbocation intermediate is the rate determining step.
The detailed mechanism of the above reaction asked is given below
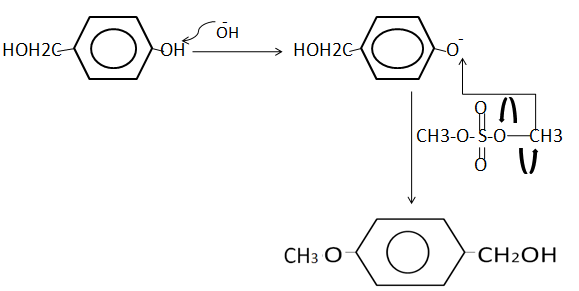
Note :
We have several types of reactions, like substitution reaction, elimination reaction, addition reaction, rearrangement reactions. One should be very careful when opting for the mechanisms, as the product of one mechanism may not be in the same stereo arrangement in another reaction mechanism. Further the preconditions like catalyst, temperature, pressure, etc vary in all the reactions.
