Question
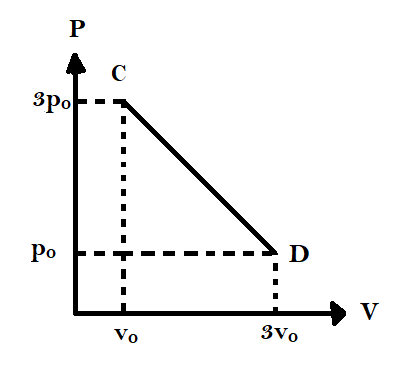
Question: The process \(CD\) is shown in the diagram. As the system is taken from \(C\) to \(D\), what happens...
The process CD is shown in the diagram. As the system is taken from C to D, what happens to the temperature of the system?
A. Temperature first decreases and then increases
B. Temperature first increases and then decreases
C. Temperature decreases continuously
D. Temperature increases continuously
Solution
With the data given to us, we can easily calculate the conditions at both the ends of CD using the ideal gas relations. However, to find what happens during the process, we can arbitrarily choose a point, such as the midpoint of CD and evaluate the conditions at that point.
Formulas used: PV=nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the universal gas constant and T is the absolute temperature.
Complete step by step answer:
-From the ideal gas law, we have:
PV=nRT⇒T=nRPV
Where P is the pressure, V is the volume, n is the number of moles, R is the universal gas constant and T is the absolute temperature.
-At point C, we have P=3p0 and V=v0
Substituting these values, we have:
TC=nR3p0v0 ………………… (1)
Where TC is the temperature at the point C
-At point D, we have P=p0 and V=3v0
Substituting these values, we get:
TD=nR3p0v0 ……………..…. (2)
Where TD is the temperature at the point D
Now let us evaluate the temperature at the midpoint of CD.
-As we are choosing the midpoint, both pressure and volume at the midpoint will be the average of pressure and volume at the two ends of CD
-Therefore, at the midpoint, P=23p0+p0
On solving this, we get:
P=24p0=2p0
Similarly, volume at the midpoint, V=2v0+3v0
On solving this, we get:
V=24v0=2v0
Substituting these values, we get:
Tmidpoint=nR2p0×2v0=nR4p0v0 ………………… (3)
From equations (1), (2) and (3) we can clearly see that temperatures at C and D are equal and the temperature at the midpoint is higher than either of these.
Hence, temperature first increases from C till the midpoint and then decreases till D.
Therefore, the correct option is B.
Note: Note that we have taken the conditions at the midpoint as the average value of both the ends of CD only because the process CD is seen to be a linear function, that is, a straight line. If the shape of the graph were to be different, we would have to use integral methods to evaluate the conditions at the midpoint. The initial increase in temperature is due to the fact that the pressure is continuously reducing and the volume is continuously increasing, till they reach a maximum at the midpoint. After the midpoint, the fall in pressure is drastic and this leads to decrease in temperature.
