Question
Question: The principal product of the reaction between methyl butanoate and 2 mole of \(C{H_3}MgBr\) after hy...
The principal product of the reaction between methyl butanoate and 2 mole of CH3MgBr after hydrolysis is:
A. C3H7COCH3
B. C3H7C(OH)(CH3)2
C. C3H7CHOCH3
D. C3H7COCO(CH3)2
Solution
In this reaction ester is converted to tertiary alcohol on reacting with two moles of Grignard reagent and after on hydrolysis. This reaction takes place in three steps, in first step methyl butanoate reacts with one mole of Grignard reagent and in the second step reacts with second mole of Grignard reagent and in the third step hydrolysis takes place.
Complete step by step answer:
In the given reaction, first reaction between methyl butanoate CH3CH2CH2COOCH3 with one mole of CH3MgBr(Grignard reagent) takes place. The Grignard reagent CH3−Mg+Br−. The negative charge of the bromine ion attack the oxygen atom of methyl butanoate and MgBr gets attached to the oxygen atom, the double bond of C=O of methyl butanoate shift to oxygen to form a positive charge on the carbon and methyl anion of Grignard reagent attack the carbon cation and methyl group gets attached to it. Again the intermediate C3H7C(CH3)OMgBrOCH3 is reacted with one mole of Grignard reagent CH3MgBr. Again the grignard reagent dissociates into CH3−Mg+Br−. The negative charge of the bromine ion attacks the oxygen atom of intermediate and MgBr gets attached to the oxygen atom. The C-O breaks down to form carbon cation and OMgBr is removed. The negative charge to the methyl anion attack the carbon cation and methyl group gets attached to it forming second intermediate C3H7C(CH3)(OMgBr)CH3. After that the second intermediate on hydrolysis where the hydrogen ion gets attached to the oxygen and remove MgBrOH to form C3H7C(OH)(CH3)2 as a main product.
The reaction of methyl butanoate with 2 mole of CH3MgBr is shown below.
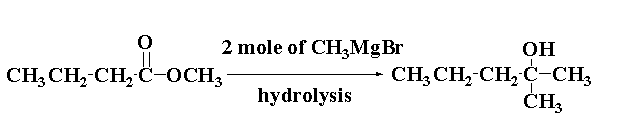
Thus, methyl butanoate CH3CH2CH2COOCH3 reacts with 2 mole of Grignard reagent CH3MgBr and on hydrolysis gives 2-methyl-pentan-2-ol C3H7C(OH)(CH3)2.
Therefore, the correct option is B.
Note: The methyl butanoate contains four carbon atoms as a hydrocarbon chain but the product 2-methyl-pentan-2-ol contains five carbon atoms as the hydrocarbon chain. The tertiary alcohol is defined as the alcohol where the hydroxyl group is attached to the carbon atom which is attached to three other carbon atoms.
