Question
Question: The pressure-volume work for an ideal gas can be calculated by using the expression \(w=\int\limits_...
The pressure-volume work for an ideal gas can be calculated by using the expression w=vi∫vfPexdv
The work can be calculated from the PV-plot by which the area under the curve within the specified limits.
When an ideal gas is compressed a) reversibly or b) irreversible from volume Vi to Vf; choose the correct option.
A) W(reversible)=W(irreversible)
B) W(reversible)⟨W(irreversible)
C) W(reversible)⟨W(irreversible) W(reversible)⟩W(irreversible)
D) W(reversible)=W(irreversible)+PexΔV
Solution
A reversible process happens in a very slow rate ,through a series of equilibrium states such that both the system and surroundings are in equilibrium.
- In an irreversible process the system may not be uniform.
Complete Solution :
In the question it is given that an ideal gas compression is taking place and the pressure –work equation is given and says which statement is true for the reversible and irreversible compression process of gas.And it is also given that the work can be calculated from the area under the P-V graph.
- So now lets see what reversible and irreversible processes are and what are the nature of the P-V plots of reversible and irreversible processes.
- Reversible processes - it is a process in which the a small change could change the process and it may be reversed.Reversible processes always proceeds very slowly by a series of equilibrium states ,ie there will be no change in the system and surroundings and the system and surrounding will be in equilibrium.
Work Done by the reversible process of the system will be minimum ,since the energy is not lost much.
The P-V plot of reversible process is:

- Irreversible process-the processes other than the reversible ones could be taken as the irreversible processes and the entropy of irreversible process increase.In irreversible process the system is not continuous ie it is not in equilibrium and the work done by the irreversible will be maximum.
The P-V plot of the irreversible processes is:
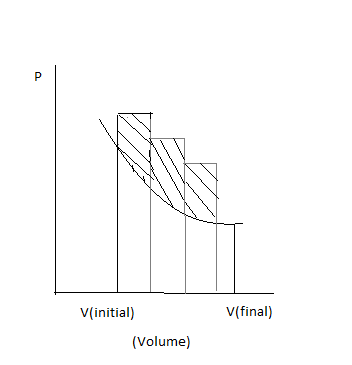
So by comparing the areas under the P-V plots of irreversible and reversible plot, we can conclude that the area curve under the P-V plot is more and hence the statement true for the question is option (B)
Work done by the irreversible process will be more. Then the work done by the reversible processes.
So, the correct answer is “Option B”.
Note: The conditions for the reversible and irreversible process should be known and the equations relating the P and V also should be known as the negative and positive sign for various cases.
