Question
Question: The presence of excess sodium in sodium chloride makes the crystal appearance yellow. This is due to...
The presence of excess sodium in sodium chloride makes the crystal appearance yellow. This is due to the presence of-
A.Schottky defect
B.Frenkel defect
C.F-centres
D.Interstitial defects
Solution
A compound imparts color due to the presence of electrons. Sodium chloride has a cubic closed packed arrangement. In sodium chloride, an electron occupies an interstitial position that when excited impart yellow color. This is due to a non-stoichiometric defect.
Complete step-by-step answer: When sodium chloride is heated in the atmosphere of sodium vapors, the sodium atom gets deposited on the surface of the crystal. The Cl− ions form the crystal lattice leave their sites and diffuse into the surface. Here, they tend to combine with sodium atoms which in turn get ionized to form Na+ ions and electrons that are released are trapped in order to maintain the electrical neutrality of the NaCl crystal. This helps the crystal to maintain their neutral character.
The electrons also absorb radiations corresponding to a certain color from white color in the following way-
They get excited and start vibrating.
These vibrating electrons emit radiations corresponding to yellow color as sodium chloride acquires a little yellow color.
These electrons are known as F-centres. Here F means Farbe (color) which is a german word.
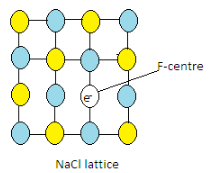
Hence due to the presence of F-centres, the crystals of NaCl are yellow-colored.
The correct answer is option C.
Note: In NaCl, the Schottky defect is also present but it is a stoichiometric defect. This defect arises due to the missing of certain ions from the crystal lattice. Vacancies or holes are created in place of the missing ions. But this defect is not the reason for the color of the NaCl crystals.
