Question
Question: The potential energy of an electron in the hydrogen atom is \( - 6.8eV.\)Indicate in which excited s...
The potential energy of an electron in the hydrogen atom is −6.8eV.Indicate in which excited state, the electron in present?
A. First
B. Second
C. Third
D. Fourth
Solution
Before answering the question let us first understand about the potential energy of electrons. The motion of electrons gives atoms their kinetic and potential energy. When electrons are excited, they travel away from the atom to a higher energy orbital. The potential energy is released in the form of kinetic energy when the electron returns to a low energy state.
Formula used:
The potential energy of an electron is calculated using the formula
En=−13.6nZ2,
where Z is the atomic number and n is the shell.
Complete step by step answer:
The cumulative energy of the electrons in an excited-state atom can be reduced by passing one or more electrons to different orbitals. By transferring an electron from a 2P orbital to a 2S orbital, the total energy of the electrons in this carbon atom can be reduced. Let us see the energy state of the hydrogen atom.
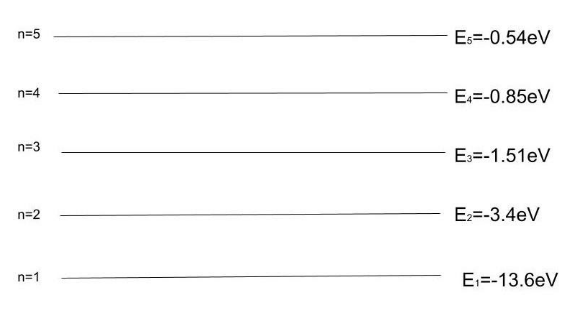
We know that energy in the En is equal to the half of the potential energy of the electron.
En=21P.E
⇒En=2−6.8eV ⇒En=−3.4eV
So the second energy state of the hydrogen atom has energy −3.4eV.
\therefore n = 2 \\\
Hence, the correct answer is option B.
Note: Let us get some more information about the energy state of hydrogen. Bohr calculated the energy of an electron in the nth energy level of hydrogen by holding electrons in circular, quantized orbits around the positively charged nucleus.
