Question
Question: The plots of \(\dfrac{1}{{{X_A}}}\;\rm{vs}\;\dfrac{1}{{{Y_A}}}\)(where \({X_A}\)and \({Y_A}\)are the...
The plots of XA1vsYA1(where XAand YAare the mole fraction of liquid A in liquid and vapor respectively) is linear with slope and intercepts respectively.
(A) PA0/PB0 and PB0(PA0−PB0)
(B) PA0/PB0 and PB0(PB0−PA0)
(C) PB0/PA0 and PB0(PA0−PB0)
(D) PB0/PA0 and PB0(PB0−PA0)
Solution
This question can be explained on the basis of Raoult’s law. The law states that partial pressure of each component of an ideal mixture of liquid is equal to the vapor pressure of pure components multiplied by its mole fraction.
Let liquid mixture has two components A and B.
Partial pressure of A is PA=PA0xA
Partial pressure of B is PB=PB0xB
PA0=vapor pressure of liquid A.
xA=mole fraction of A.
PB0=vapor pressure of liquid B.
xB=mole fraction of B.
Complete Step by Step Solution:
In the given equation:
xA=mole fraction of A in liquid state
yA=mole fraction of A in vapor phase
If we plot a graph of xA1vs yA1we get a straight line.
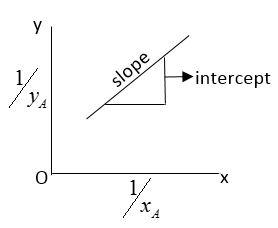
Let PA0be partial pressure of liquid A and PB0partial of vapor.
By Raoult’s law we have,
PA=PA0xA_____ (1)
PB=PB0xB_____ (2)
And PA1=PMYAand PB1=PmYB
∴YAPA=YBPB1
⇒yAPA0xA=yBPB0xB=(1−yA)PB0(1−xA)
As we know the sum of mole fraction of xAand xBis 1.
xA+xB=1 and yA+yB=1
⇒xB−(1−xA)
⇒yB−(1−yA)
⇒xAPB0=yAPA0+(PB0−PA0)
⇒xA1=yA1.PB0PA0+(PB)(PB0−PA0)
Comparing above equation with y=mx+c we get
y=xA1
x=yA1
m=PB0PA0
c=(PBPB0−PA0)
Here, y and x are variable
m=slope=PB0PA0
c=intercept=[PBPB0−PA0]
Therefore, from the above explanation the correct option is (D) PB0/PA0 and PB0(PB0−PA0)
Raoult’s law is used to estimate the contribution of an individual component of a liquid or solid mixture to total pressure exerted by the system.
Note:
We should know that Raoult’s law is applicable to solutions containing non-volatile solute. It is not applicable for solute which dissociate or associate in the solution.
