Question
Question: The plot of log k vs. 1/T helps to calculate: A. The energy of activation. B. The rate constan...
The plot of log k vs. 1/T helps to calculate:
A. The energy of activation.
B. The rate constant of the reaction.
C. The order of the reaction.
D. The energy of activations as well as the frequency factor.
Solution
Use the Arrhenius equation related to log k and 1/T. Interpret the slope and intercept of the plot of log k vs 1/T.
Formula used:
Arrhenius equation
logk=logA−2.303REa×T1
Complete step by step answer:
According to Arrhenius theory non-active or non-reactive molecules can become activated by absorption of heat energy.
The Arrhenius equation gives the relationship between the rate constant k and temperature.
logk=logA−2.303REa×T1
Where,
k = rate constant
A = frequency factor
Ea= energy of activations
R= gas constant
T = temperature
The plot of logk vs 1/T is as follows:
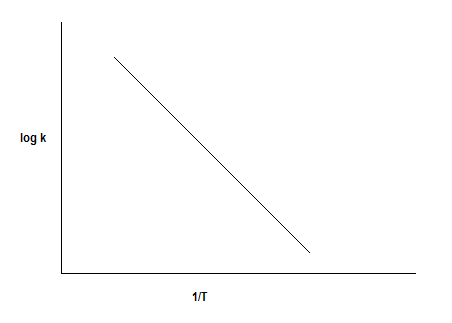
The linear equation for a straight line is y=mx+c
Where,
y=logk
x=T1
Slope = m=−2.303REa
Intercept = logA
So, from the slope and intercept of the plot of logk vs 1/T we can calculate the energy of activation and frequency factor respectively.
The order of the reaction is experimental property. It can be determined from the concentration of the reactant and the rate of reaction. It cannot be determined from the plot of logk vs 1/T.
Thus, the correct option is (D) The energy of activations as well as the frequency factor.
Note: Activation energy is the minimum amount of energy required to convert the reactant to product. Frequency factor (A) is constant for the given reaction and it describes the frequency of collision of reacting molecules. Using data of rate constant at various temperatures we can calculate the energy of activation and frequency factor of the reaction from the plot of logk vs 1/T.
