Question
Question: The photo cathode and collector plate are kept \( 10cm \) and connected through a galvanometer witho...
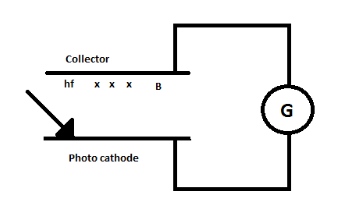
The photo cathode and collector plate are kept 10cm and connected through a galvanometer without a battery. A magnetic field B exists parallel to the plates. The work function of the emitter is 2.39eV and the light incident on it has wavelength 400 to 600nm . Find the minimum value of B so that the galvanometer shows null deflection.
Solution
In order to solve this question, we are going to first consider the condition at which the galvanometer is going to show no deflection at all. After that, taking Einstein's equation, we are going to find the value of maximum kinetic energy, after which the value of the minimum magnetic field is found.
According to the Einstein’s equation,
(KE)max=hf−ϕ
The radius for the revolution is given by
r=qBmv
Complete step by step solution:
The galvanometer will indicate zero deflection if the electrons complete the semi-circular path before reaching the plate P . The photoelectric effect is seen only for the wavelength 400nm .
Now, according to the Einstein’s equation,
(KE)max=hf−ϕ
Where, hf is the energy and ϕ is the work function of the emitter which is equal to 2.39eV
Putting these in the equation above, we get
\left( {KE} \right)\max = 6.626 \times {10^{ - 34}} \times 400 \times {10^{ - 9}} - 2.39eV \\\
\Rightarrow \left( {KE} \right)\max = 3.1eV - 2.39eV \\\
\Rightarrow \left( {KE} \right)\max = 0.71eV \\\
Now as we know that the radius for the revolution is given by
r=qBmv
Calculating the magnetic field from this, we get
B = \dfrac{{\sqrt {2 \times 9.1 \times {{10}^{ - 31}} \times 0.71 \times 1.6 \times {{10}^{ - 19}}} }}{{1.6 \times {{10}^{ - 19}} \times 1}} \\\
B = 2.842 \times {10^{ - 5}}T \\\
Thus, the minimum value of B for which the galvanometer shows no deflection is 2.842×10−5T.
Note:
The energy of the photons is given corresponding to the light of the wavelength 400nm and 600nm . The photoelectric emission is possible only with the wavelength λ1 . Photoelectrons experience magnetic force and move along a circular path and the zero deflection is seen when the electron is in the middle of the path.
