Question
Question: The pH of the digestive juices within the human small intestine is between 7.5 and 8.5. This environ...
The pH of the digestive juices within the human small intestine is between 7.5 and 8.5. This environment is slightly
a) Basic
b) Acidic
c) Neutral
d) None of the above
Solution
pH 7.5-8.5 is a high pH value which corresponds to low H+ ions. Also, acidic and basic environments have high and low concentrations of H+ ions respectively.
Complete answer:
The human small intestine has a slightly basic environment with the pH of the digestive juices between 7.5 and 8.5. The quantitative measure of the acidity and basicity of an aqueous solution is termed as pH. It refers to the relative concentration of H+ ions in a solution. Low pH values indicate high concentrations of H+ ions (acids) and high pH values indicate low concentrations.
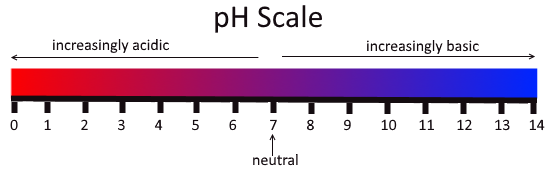
Fig: The pH scale
The values of the concentration of the hydrogen ion ranging between about 1 and 10−14 gram-equivalents per liter alternately between 0 and 14 are defined by the pH. In pure water the pH is 7 that means the concentration of the hydrogen ion is 10−7 gram-equivalents per liter. Pure water, pH 7 is neither acidic nor basic, it is neutral. A solution with a pH less than 7 is considered acidic whereas a solution with a pH greater than 7 is considered basic, or alkaline. A scale which measures from 0 to 14, and indicates just how acidic or basic a substance is is called pH scale. The pH of a substance is given by
pH=−log[H+]
pOH=−log[OH−]
where [H+]denotes the molar hydrogen ion concentration,
[OH−] denotes the molar hydroxide ion concentration
Also, pH+pOH=14
So, the correct answer is ‘Basic’.
Note:
- pH stands for potential of hydrogen.
- Danish biochemist Soren Peter Lauritz Sorensen devised the pH scale in 1923.
