Question
Question: The pH indicators are A.Salts of strong acids & strong bases B.Salts of weak acids & weak bases ...
The pH indicators are
A.Salts of strong acids & strong bases
B.Salts of weak acids & weak bases
C.Either weak acids or weak bases
D.Either strong acids or strong bases
Solution
pH indicators are compounds which manifest whether a solution is acidic or basic. The basic criterion of a good indicator is the ability to change its color from the acidic to the basic medium and vice versa.
Complete step by step answer:
This is a very conceptual question which demands the understanding the concept of equilibrium between the acidic and basic forms of an indicator. You also need the understanding of Le Chatelier’s principle.
Firstly, let’s look at Le Chatelier’s principle. It states that when the equilibrium of a system is disturbed then the equilibrium shifts to that side so as to minimize the change.
An indicator can be written by a general formula of:
HInd+H2O⇄H3O++Ind−
Where HInd is the acidic form of the indicator and Ind− is the basic form of the indicator. During a titration, as the H3O+ of the solution increases or decreases, it shifts the reaction to the left or right according to the Le Chateleir’s principle and a time will come when there are enough HInd molecules or Ind− molecules in the solution that it will show its corresponding colour. It is known as the end point.
Now, you have enough knowledge to choose the indicators. Since, different reactions require different ranges hence, either weak acids or weak bases are perfect to choose as indicators because you can’t get millions of different indicators each for a specific reaction. Moreover, the equilibrium concept also works perfectly for weak acids and bases. So the answer to this reaction is option C. Let’s look at an example:
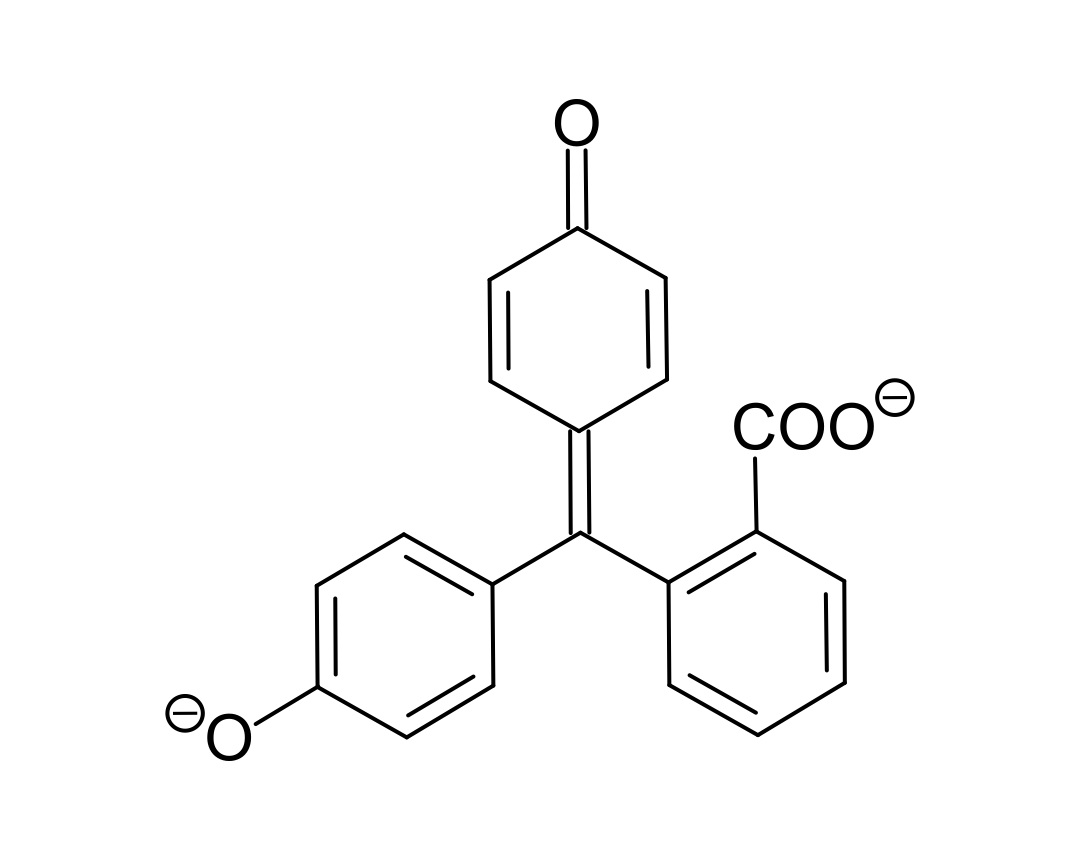
It is a basic form of the indicator Phenolphthalein.
So, the correct answer is Option C.
Note:
Strong acids or bases are not chosen as indicators since they would not have a range for the change of color in acid and basic form. The end point would be much abrupt then and it would be much harder to get an accurate value for the titration.
