Question
Question: The percentage of free \(S{{O}_{3}}\) in oleum sample which is labelled as 106% is: A. 55% B. 26...
The percentage of free SO3 in oleum sample which is labelled as 106% is:
A. 55%
B. 26.67%
C. 38%
D. 43.33%
Solution
As we know that the molecular formula of oleum is H2SO4.SO3, which is produced by contact process. It is found that oleum acts as an intermediate in production of sulphuric acid. The structure of Fuming sulphuric acid is:
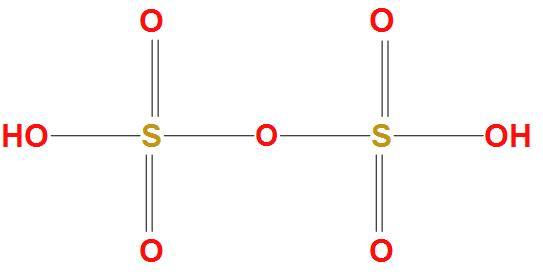
Complete answer:
- As we know that the formula of oleum is H2SO4.SO3 .
- When we will add water in H2SO4.SO3 it will form H2SO4 as the water molecule is found to react with SO3, and will give H2SO4.
- 106% labelled oleum means 100 g oleum will react with H2O to form 106% H2SO4.
- Therefore, we can say that the mass of H2O = 106 g - 100 g = 6 g.
- Actually, as we have discussed that water molecule is found to react with SO3, and will give H2SO4. Here, 1 mole of SO3 reacts with 1 mole of water to form 1 mole of sulphuric acid.
The molar mass of SO3 , H2O and H2SO4 is 80 g, 18 g , 98 g. We can calculate them as: molar mass of SO3:
