Question
Question: The pair(s) of reagents that yield paramagnetic species is/are : a.) Na and excess of \(N{H_3}\) ...
The pair(s) of reagents that yield paramagnetic species is/are :
a.) Na and excess of NH3
b.) K and excess of O2
c.) Cu and dilute HNO3
d.) O2 and 2 - ethylanthraquinone
Solution
The species in the options combine to give products. Most of these form the products that have unpaired electrons and are paramagnetic. The aromatic system gives hydrogen peroxide which is diamagnetic in nature. The metals given in above options produce species with unpaired electrons.
Complete step by step answer:
First, let us know about paramagnetic species. These are those species that have unpaired electrons. Now, let us see the options given-
The first option is Na and excess of NH3. The Na ion in the liquid ammonia forms solvated electrons. These are unpaired. So, it is paramagnetic.
The second option is K and excess of O2. This combination gives superoxide KO2. This superoxide has O2− ion which has one unpaired electron. Thus, it is also paramagnetic.
The third option is Cu and dilute HNO3. The Cu reacts with nitric acid (HNO3) to form cupric nitrate. This cupric nitrate has Copper in +2 oxidation state with one unpaired electron along with NO. So, even these reagents are paramagnetic in nature. The reaction can be given as -
3Cu+8HNO3(dil)→3Cu(NO3)2+4H2O+2NO
The fourth and the last option is O2 and 2 - ethyl anthraquinone. This reaction can be written as-
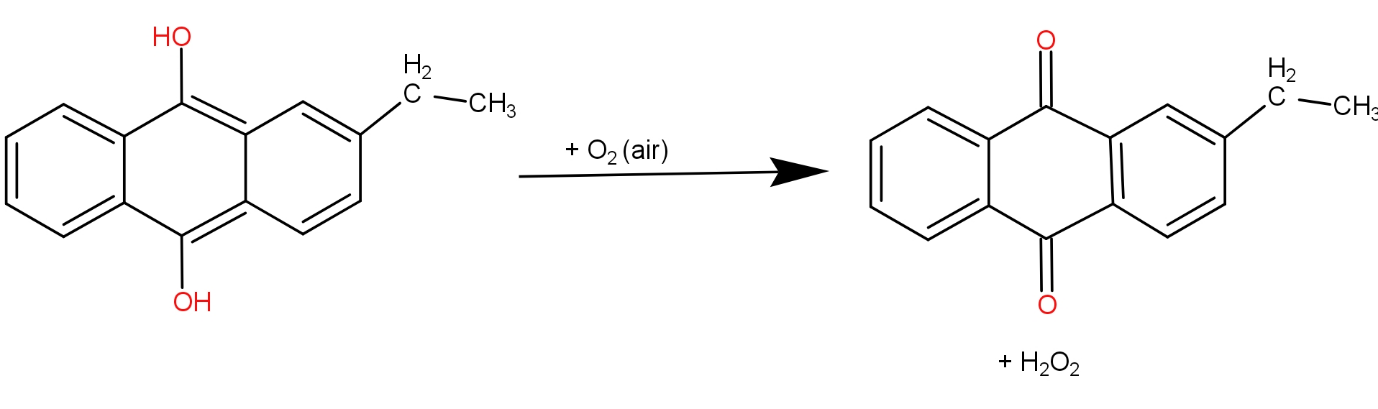
The hydrogen peroxide produced here does not have any unpaired electron. So, it is diamagnetic.
So, the correct answer is “Option A, B and C”.
Note: It must be noted that due to presence of unpaired electrons, the magnetic moment value comes out to be non-zero. These species show colours due to the presence of unpaired electrons that can get excited.
The unpaired electrons in solvated Na and ammonia can also conduct electricity.
