Question
Question: The pair of species with similar shape is (A)\({\rm{PC}}{{\rm{l}}_3}\), \({\rm{N}}{{\rm{H}}_{\rm{3...
The pair of species with similar shape is
(A)PCl3, NH3
(B) CF4, SF4
(C) PbCl2,CO2
(D) PF5, IF5
Solution
We know that valence shell electron pair repulsion (VSEPR) theory is used to determine the geometry and shape of molecules from the electron pairs surrounding the central atom. The electron pairs may be bonded pairs or lone pairs.
Complete step by step answer:
Let’s find the correct answer from the given options.
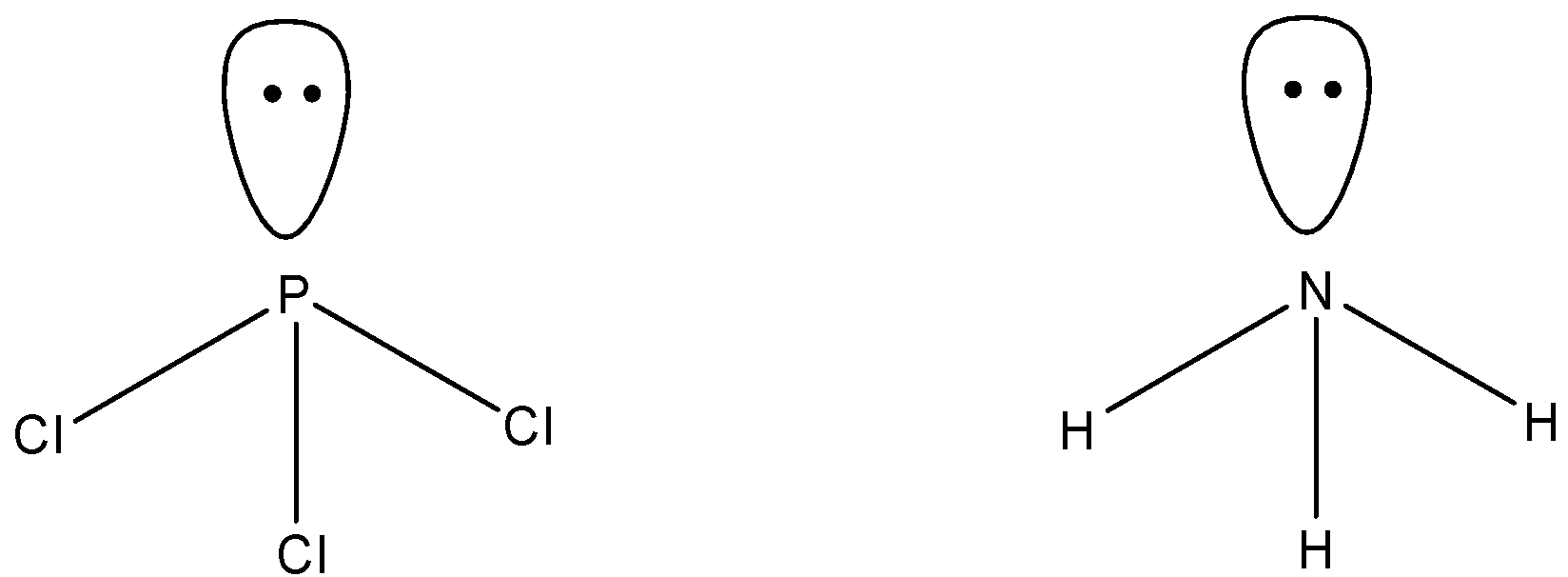
Option A says PCl3 and NH3 has similar shape. Now, we have to determine if they have similar shapes or not.
Phosphorus and nitrogen both belong to group 15 of the periodic table. So, valence electrons in both the elements are 5. In PCl3, three electrons of nitrogen are shared with three chlorine atoms. So, the number of bond pairs in PCl5 is 3 and an unshared electron pair present. Similarly in NH3 also, three bond pairs and one lone pair present. If a compound has three bond pairs and one pair, the shape is always pyramidal. So, the shape of both compounds is similar, that is pyramidal.
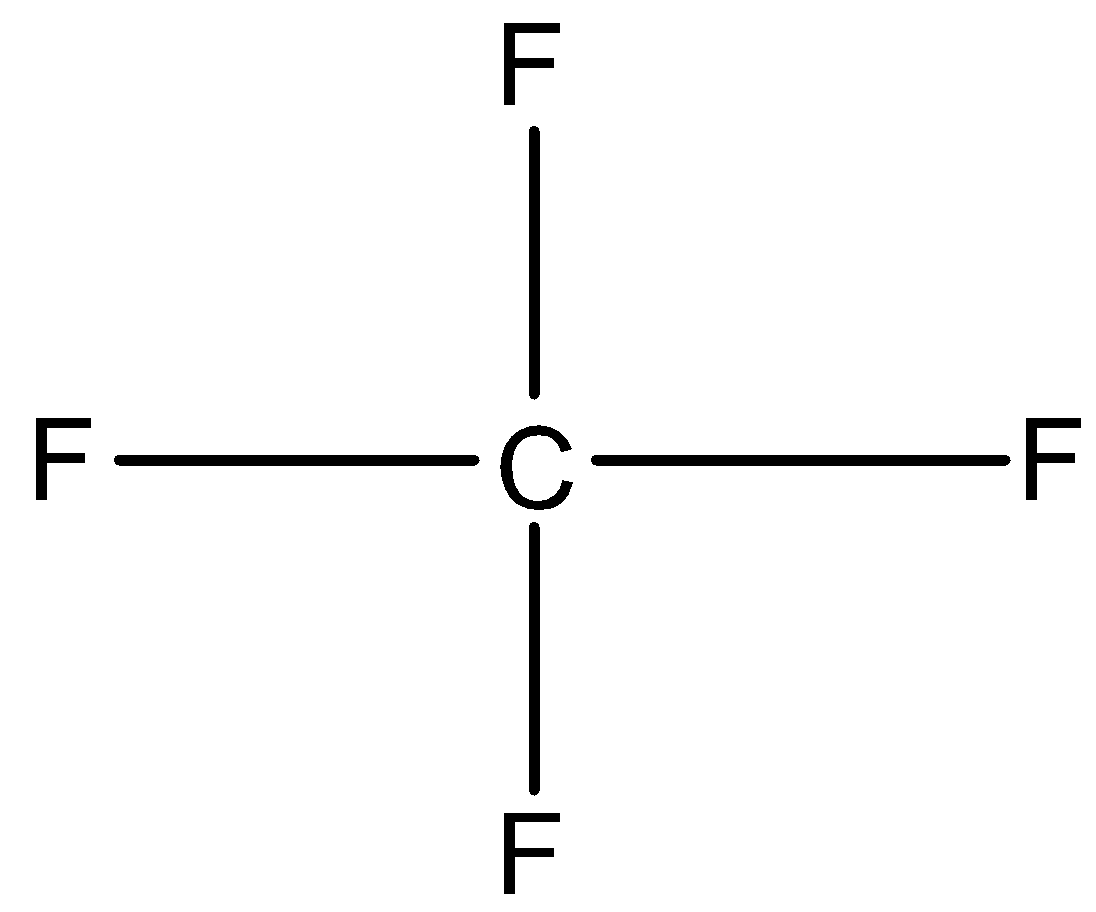
Option B is CF4 andSF4. In CF4, Carbon has four valence electrons. So, it shares its four electrons with four fluorine atoms. So, four bond pairs present in CF4. Therefore, the structure of CF4is tetrahedral.
In SF4, sulphur has six valence electrons and it forms only four bonds with four fluorine atoms. Two electrons are unshared in the compound (lone pair). So, four lone pairs and one pair is present in SF4. So, the shape of SF4 is see-saw.
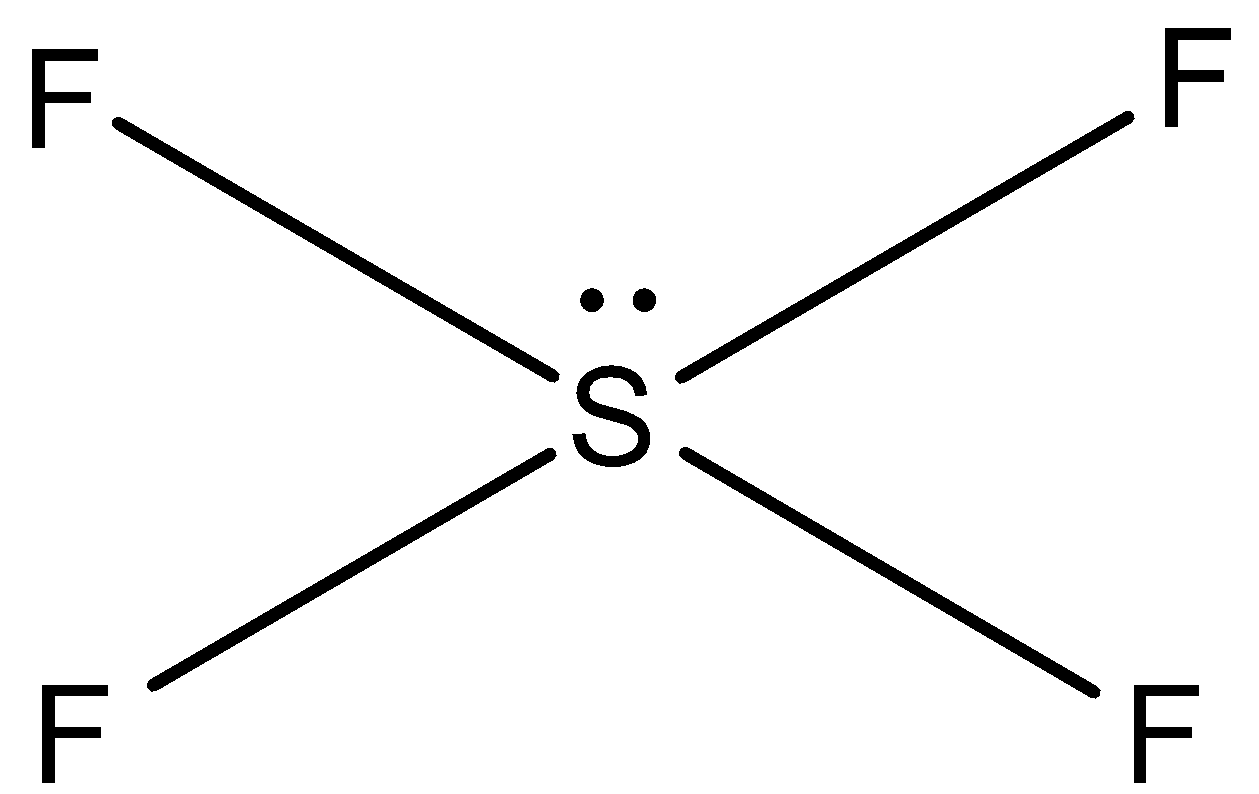
Therefore, both the compounds do not possess similar shape.
Option C is PbCl2 andCO2.
In CO2, four valence electrons of carbon are shared with two oxygen atoms. So, the compound forms two double bonds. That means, electrons groups surrounding carbon atoms are two. Therefore the structure of CO2 is linear.
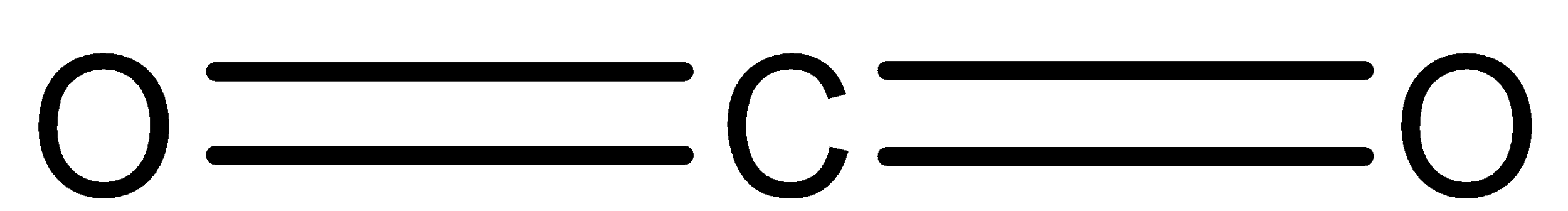
In PbCl2, Pb has four valence electrons and chlorine has valency of one. So, two electrons of Pb are shared with two chlorine atoms to form two bond pairs. And one pair of electrons is unshared. So, the structure of PbCl2 is bent.
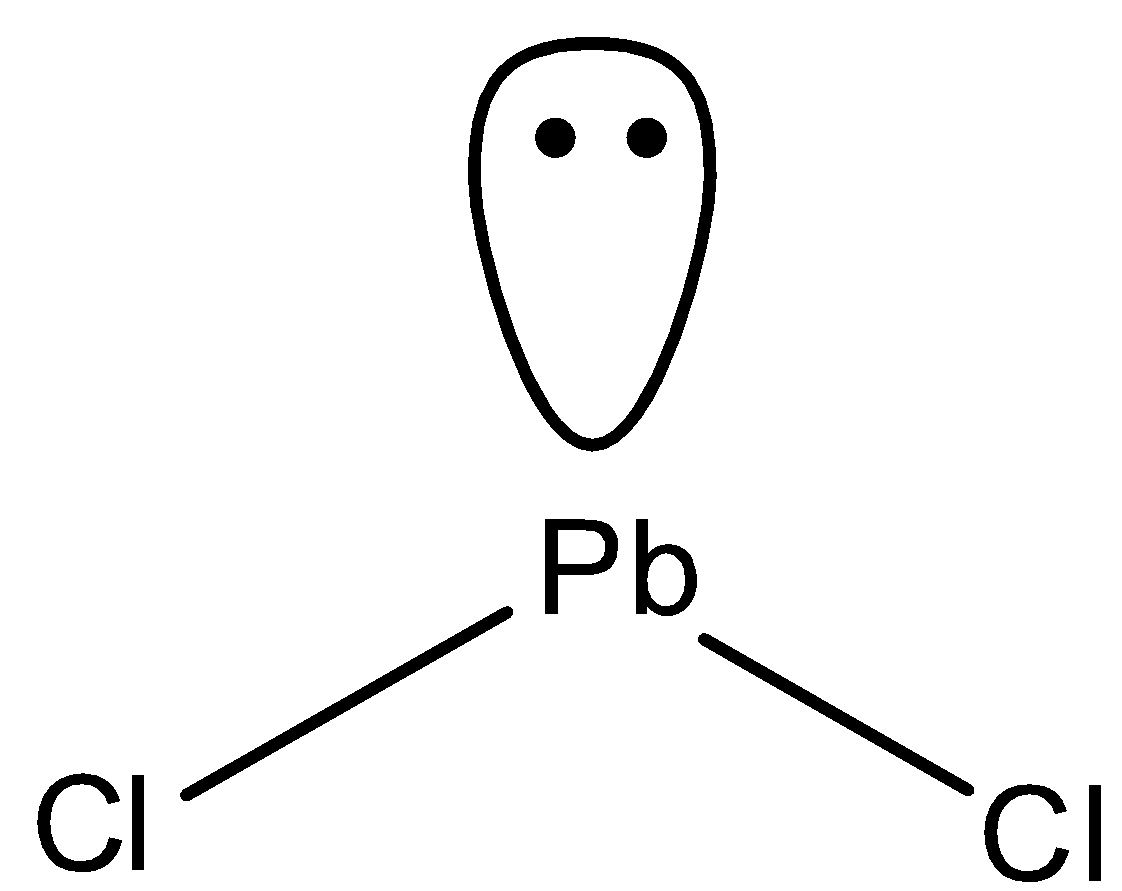
So, both PbCl2 andCO2 possess different structures.
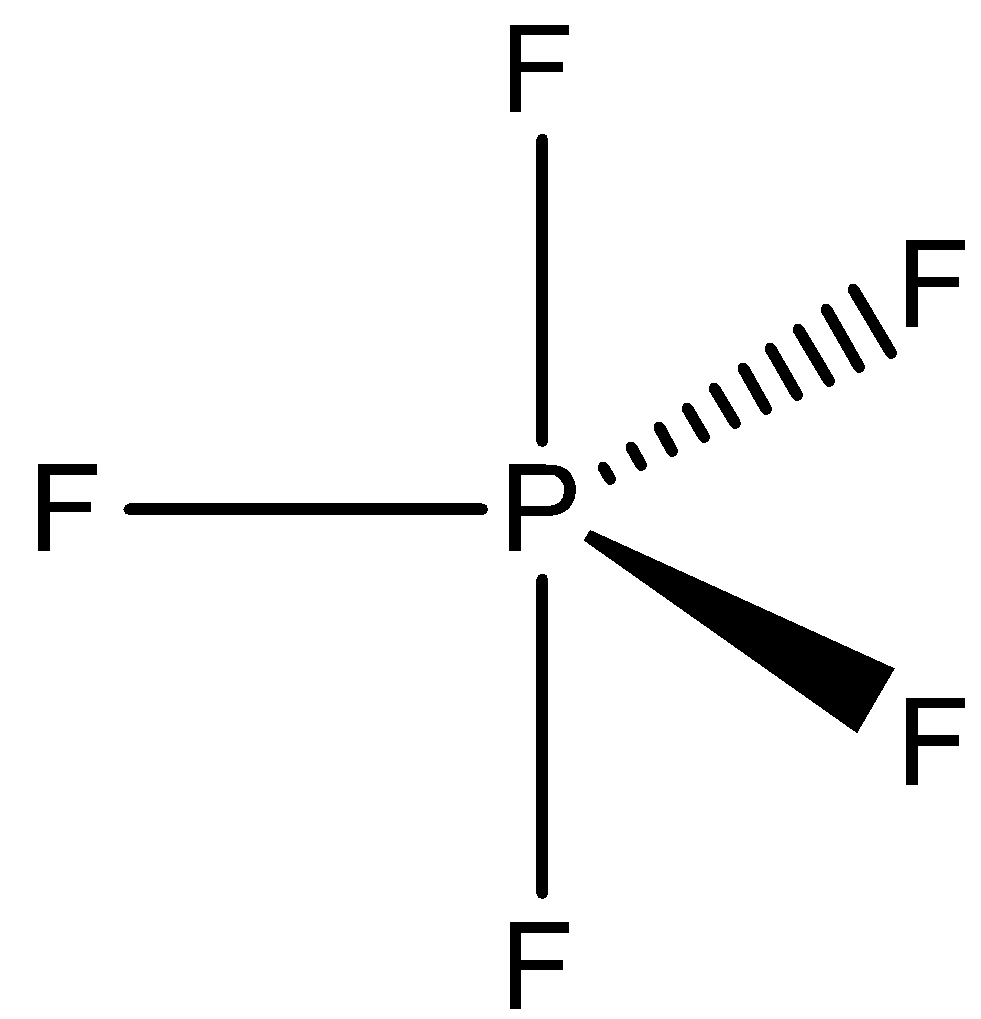
OptionD is PF5 and IF5. In PF5, phosphorus has five valence and chlorine has valency one. So, five electrons of phosphorus are shared with five fluorine atoms. So, no lone pair is present. Therefore, the shape of PF5 is trigonal bipyramidal.
In IF5, iodine has seven electrons and fluorine is one. So, five electrons of iodine are shared with five fluorine atoms and one electron pair is unshared. So, in the compound five bond pairs and one lone pair are present. Therefore, the shape of IF5is square pyramidal.
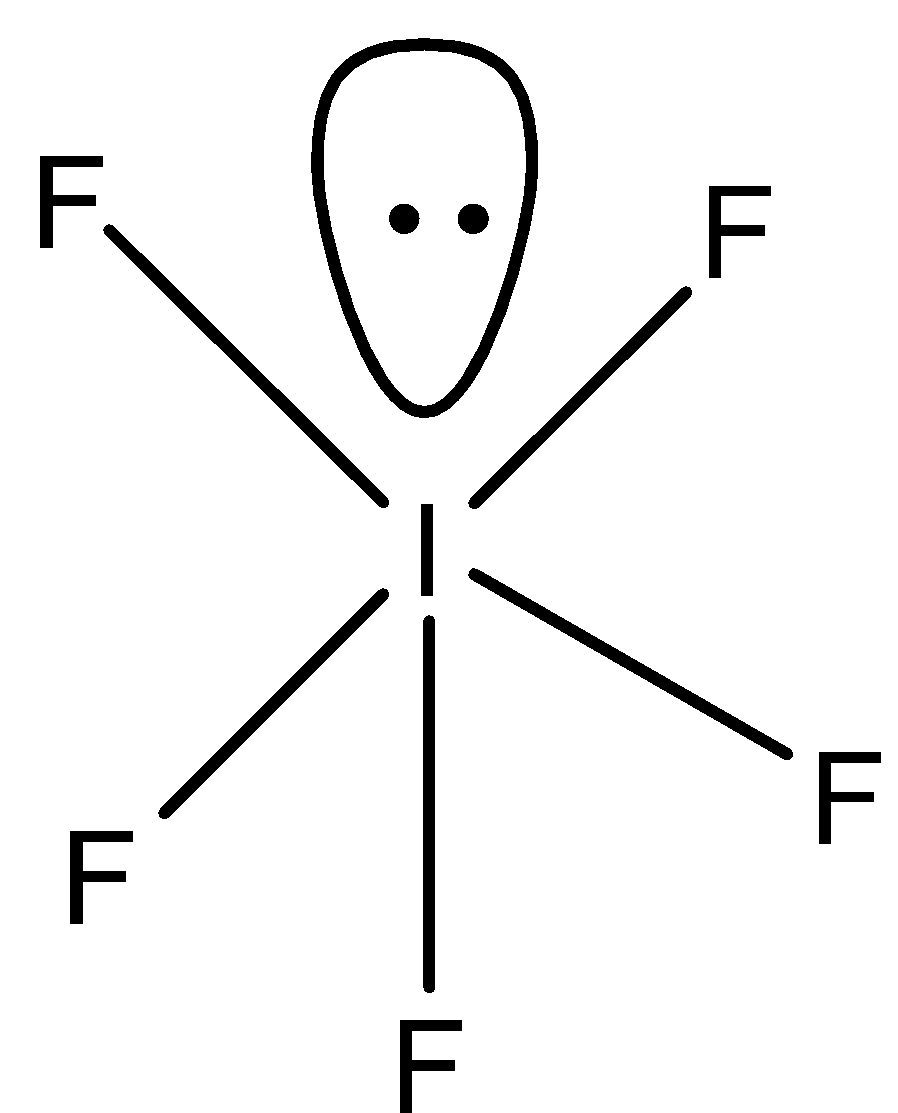
Therefore, both the compounds have different shapes.
So, the correct answer is Option A.
Note: According to VSEPR theory, lone pair electrons repel each other more strongly than that of bond pair electrons. So, the decreasing order of repulsion is lp-lp>lp-bp>bp-bp. So, repulsion between bond pair-bond pair is least and between lone pair-lone pair is highest.
