Question
Question: The packing efficiency of the face centred cubic (fcc), body-centred cubic (bcc) and simple primitiv...
The packing efficiency of the face centred cubic (fcc), body-centred cubic (bcc) and simple primitive cubic (pc) lattices follows the order:
A. fcc > bcc > pc
B. bcc > fcc > pc
C. pc > bcc > fcc
D. bcc > pc > fcc
Solution
Hint: We know that each cube has 8 corners, 12 edges, 6 faces, 12 face diagonals and 8 body diagonals. Keeping this information in mind we need to proceed for the comparison.
Step by step answer:
The percentage efficiency of a simple cubic unit cell is:
Suppose,
Length of the unit cell = a
Radius of the sphere (atom) = r
Total volume of the unit cell = a3= (2r)3= 8r3
Number of atoms per unit cell = 8 !!×!! 81 = 1
Volume of the atom \text{r = }\dfrac{\text{1}}{\text{2}\sqrt{\text{2}}\text{a}}$$$$\text{= }\dfrac{\text{4}}{\text{3}}\text{ }\\!\\!\pi\\!\\!\text{ }{{\text{r}}^{\text{3}}}
∴packing fraction = Total volumeOccupied volume
= 8r3(4/3) !!π!! r3= 0.5233
Thus, the percentage of occupied volume or packing efficiency = 0.5233 !!×!! 100 = 52.33 !!
The percentage efficiency of a body-centred unit cell is:
Suppose,
Length of the unit cell = a
Radius of the sphere (atom) = r
In this unit cell,
a = 34 !!×!! r
Total volume of the unit cell = a3= (34)3r3= 3364 r3
Number of atoms per unit cell = 2
Volume of two atoms = 2 !!×!! 34 !!π!! r3
Therefore, packing fraction (3D) = Total volumeOccupied volume
=3364r32 !!×!! 34 !!π!! r3=0.68
Thus, the percentage of occupied volume or packing efficiency = 68 !!
The percentage efficiency of a face-centred cubic unit cell:
Suppose,
Length of the unit cell = a
Radius of the sphere (atom) = r
In this unit cell,
a=22×r
Total volume of the unit cell =a3=(22)3r3=162r3
Number of atoms per unit cell = 4
Volume of four atoms = 4 !!×!! 34 !!π!! r3
(This is the occupied volume)
Therefore, packing fraction (3D) = Total volumeOccupied volume
=162r34 !!×!! 34 !!π!! r3=0.7401
Thus, the percentage of occupied volume or packing efficiency = 74.01 !!
Hence we can see that the packing efficiency goes in to order:
fcc > bcc > pc
So Option A is the correct answer.
Note:
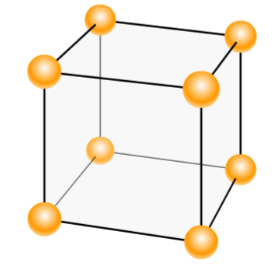
Atomic radius of simple cubic unit cell is: r = 2a
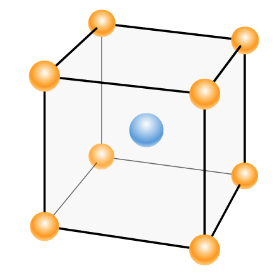
Atomic radius of body-centred unit cell is: r = 4 a3
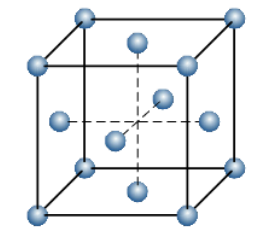
Atomic radius of face-centred unit cell is: r = 22a1
