Question
Question: The \(P - P - P\) in \({P_4}\) molecule and \(S - S - S\) angle in \({S_8}\) molecule is (in degree)...
The P−P−P in P4 molecule and S−S−S angle in S8 molecule is (in degree) respectively:
(A) 60∘,107∘
(B) 107∘,60∘
(C) 60∘,108∘
(D) 60∘,40∘
Solution
Atoms in P4 molecules are arranged in a tetrahedron structure. Whereas S8 molecules’ atoms are arranged in a crown shape. Thus, they will have different angles between their atoms corresponding to their respective structure.
Complete step by step answer:
P4 (tetra phosphorus) is a member of tetratomic phosphorus.
A nonmetal element that has the atomic symbol P, atomic number 15 and atomic mass 31. It is an essential element that takes part in a broad variety of biochemical reactions.
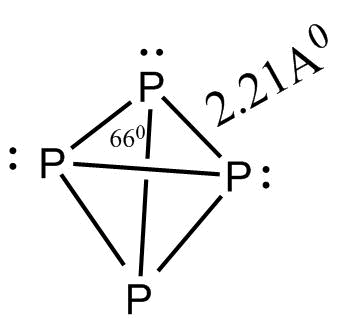
In P4 molecule the four Sp3 hybridized phosphorus atoms lie at the corners of a regular tetrahedron with P−P−P angle 60∘
P4 has following characteristics:
-Tetrahedron shape
-P-P-P bond angle is 60∘
-There are six bonds in total.
-Four lone pairs of electrons.
-Can form a single bond with other atoms S8 molecules.
Cyclo octa Sulphur is a homo monocyclic compound composed of eight Sulphur atoms. It has been isolated from Ganoderma lucidum, a mushroom commonly used in Chinese medicine. It has a role as a fungal metabolic and a bacterial metabolite. It is an elemental Sulphur and a homo monocyclic compound.
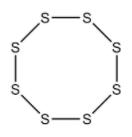
Top View
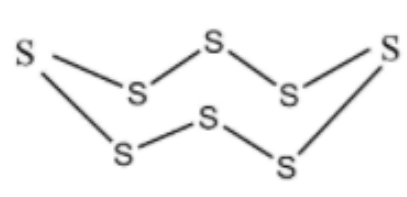
Side View
In S8 molecule, S−S−S angle is 107∘.
So, the answer is (A) 60∘,107∘.
Note: The Molecular solid is a solid consisting of discrete molecules. The cohesive forces are Van Der waal forces, dipole-dipole interaction, etc. So P4 and S8 are called molecular solids.
