Question
Question: The oxo acids of \({{P}_{2}}{{O}_{5}}\)are \({{H}_{3}}P{{O}_{4,}}{{H}_{4}}{{P}_{2}}{{O}_{7,}}HP{{O}_...
The oxo acids of P2O5are H3PO4,H4P2O7,HPO3, and H3PO3.
(A)-True
(B)-False
Solution
Phosphorus forms a number of oxoacids. The oxidation state of phosphorus in oxo acids is usually +1, +3,+4,+5. In P2O5, the oxidation state of phosphorus is+5. Composition of oxo acids depends on loss or gain of water molecules or oxygen atoms.
Complete step by step answer:
-In oxoacids phosphorus is surrounded by other atoms tetrahedrally.
-In P2O5, the oxidation state of phosphorus is +5. In oxo acids, oxygen atoms are attached to atoms.
-In oxo acids of P2O5 , phosphorus will have an oxidation state as +5 and will have phosphorus oxygen double bond.
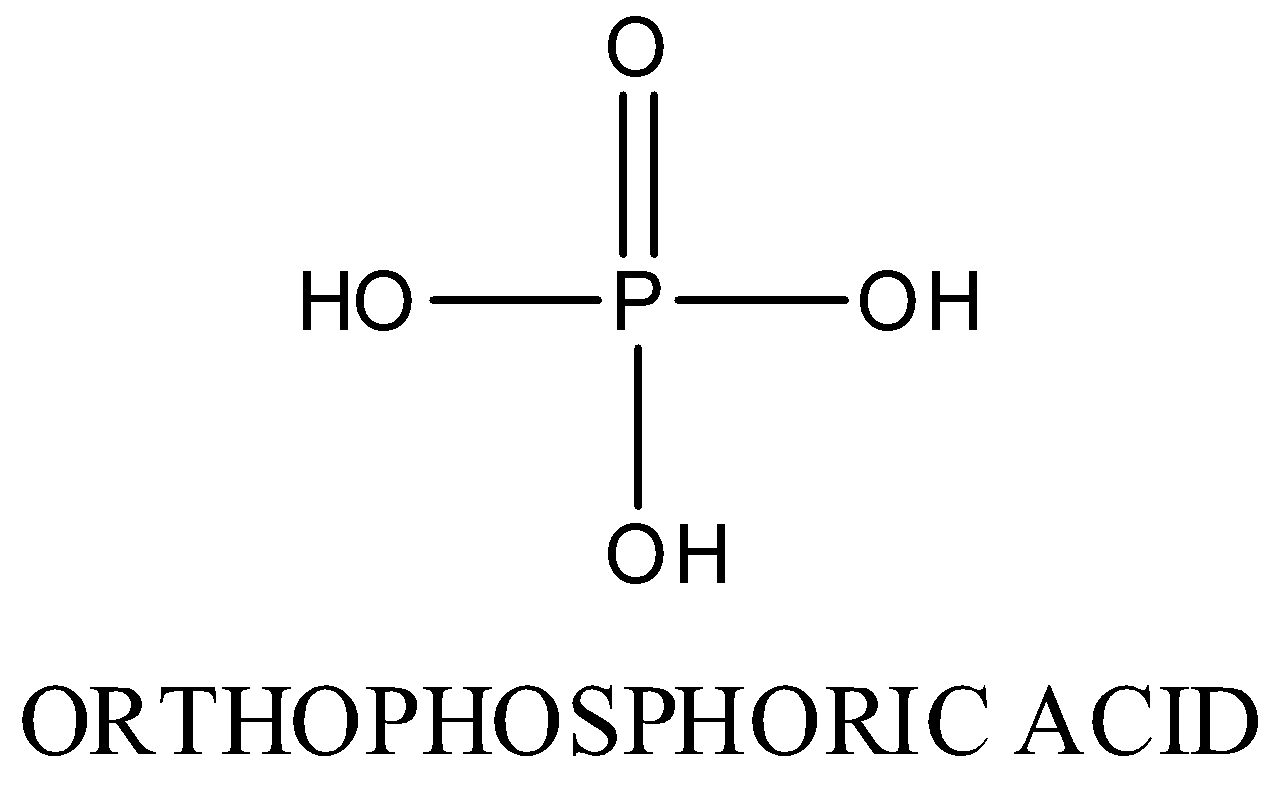
-In H3PO4,
H3PO43×1+P+4×−2=03+P+(−8)=0P=+5
Oxidation state of phosphorus is +5. Three P-OH bonds and one P=O bond is present
The structure is as following:
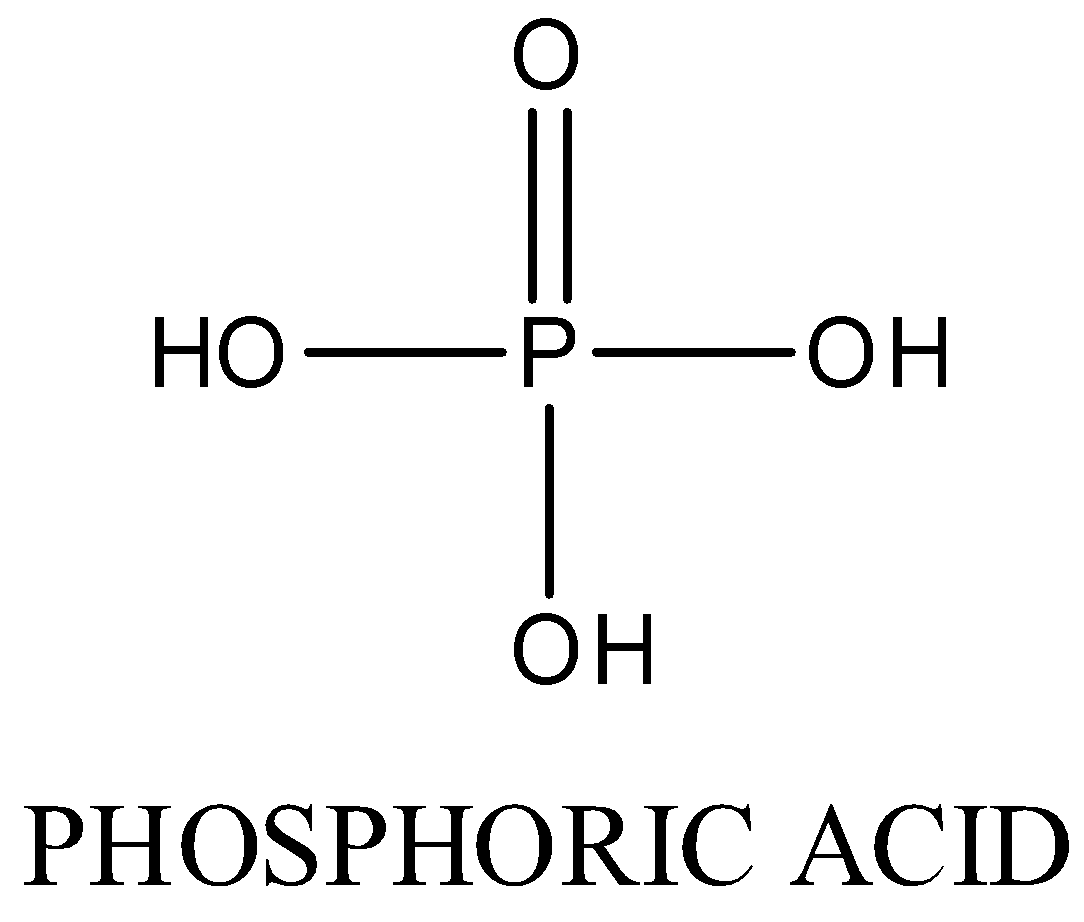
In H4P2O7, Oxidation state of phosphorus is +5. Four P-OH bonds , two P=O bond and one P-O-P bond is present. The structure is as following:
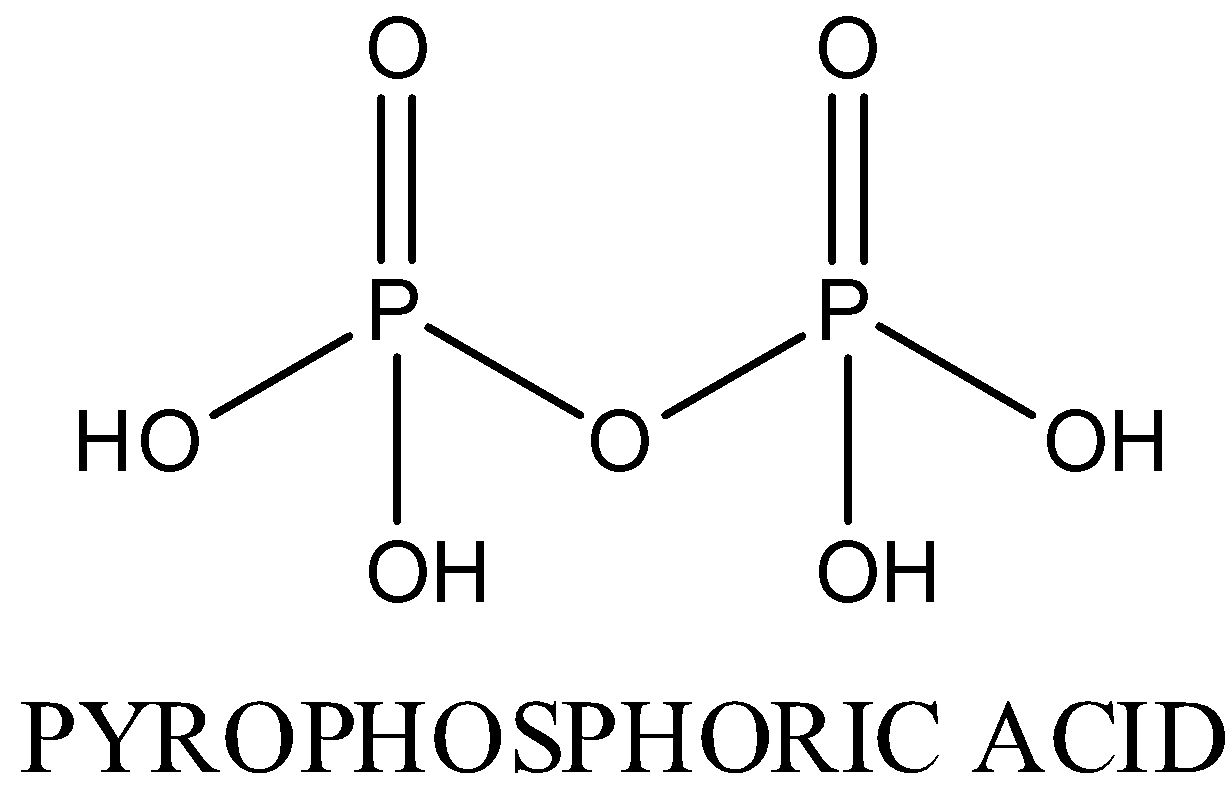
In HPO3, oxidation state of phosphorous is +5.It exists in polymeric form.The structure is as following:
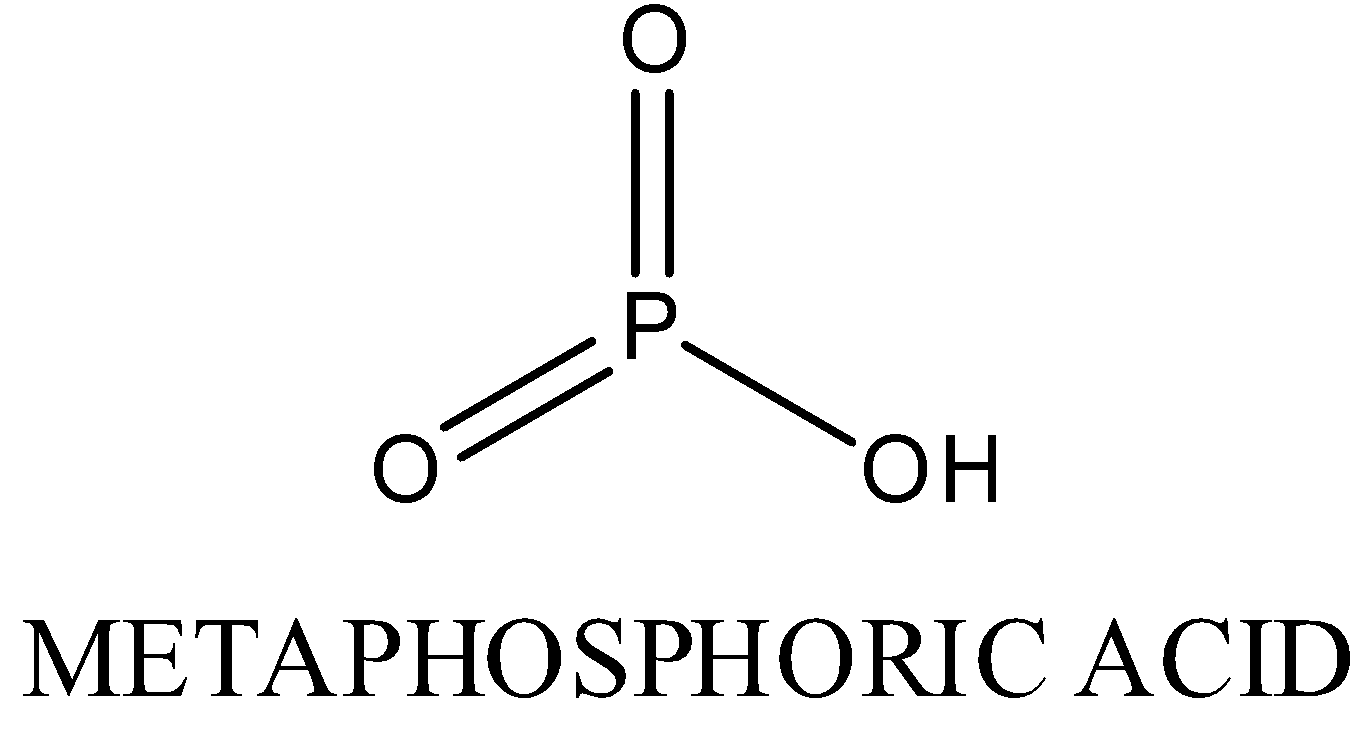
In H3PO3 , oxidation state of phosphorous is +3,Two P-OH bonds, one P=O bond, one P-H bond is present.
Structure is as following:
In H3PO3, oxidation number of phosphorus is +3, so it is not an oxo acid of P2O5.
Hence, statement The oxo acids of P2O5 are H3PO4,H4P2O7,HPO3 and H3PO3 is false.
So, the correct answer is “Option B”.
Note: Phosphorus pentoxide is produced by treating phosphorus with excess of oxygen. Phosphorus undergoes combustion to form phosphorus pentoxide.It is anhydride of orthophosphoric acid H3PO3 H4P2O7 is pyrophosphoric acid. HPO3 is metaphosphoric acid. Oxoacid is an acid that contains oxygen atoms.
