Question
Question: The oxidation states of iodine in \[{\rm{HI}}{{\rm{O}}_{\rm{4}}}\], \[{{\rm{H}}_{\rm{3}}}{\rm{I}}{{\...
The oxidation states of iodine in HIO4, H3IO5and H5IO6 are respectively
(A) +1, +3, +7
(B) +7, +7, +3
(C) +7, +7, +7
(D) +7, +5, +3
Solution
As we know that the oxidation states usually referred to the charge of any chemical compound. For example, an oxygen atom is an electronegative atom and has −2 charge due to only six electrons in its outermost orbital.
Complete answer:
The oxidation state of any atom is the state when that atom loses its electrons. In the above question, there are three compounds in which iodine loses its electrons. As we know that iodine is 17th group element and it has seven electrons in its outermost shell so it can lose maximum of seven electrons.
Let’s calculate the oxidation of iodine in these compounds.
In HIO4,
Suppose x is the oxidation state of iodine, it is calculated as follows;
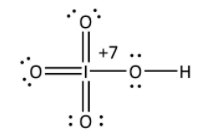
Here, the formal charge of hydrogen is and formal charge of oxygen is −2 and represented as-
In H3IO5,
Suppose x is the oxidation state of iodine, it is calculated as follows;
And represented as-
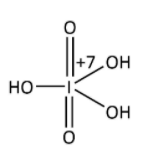
In H5IO6,
Suppose x is the oxidation state of iodine, it is calculated as follows;
Therefore, the oxidation state of iodine in all of three compounds is +7.
The compound is represented as-
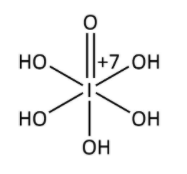
Hence, the correct answer is option (C).
Note: Any compound in which the central element exists with its highest oxidation state, will have the maximum oxidizing property such as HIO4, H3IO5and H5IO6. These are said to be oxidizing agents.
