Question
Question: The oxidation state of Fe in \[F{{e}_{3}}{{O}_{4}}\] is: a.) +3 b.) +(8/3) c.) +6 d.) +2...
The oxidation state of Fe in Fe3O4 is:
a.) +3
b.) +(8/3)
c.) +6
d.) +2
Solution
For finding the oxidation state of Fe we will draw the structure of given compoundFe3O4 it will be easy for us to find the oxidation state. Then we will see the oxidation states of oxygen and then according to the oxidation state of oxygen we will find the oxidation state of Fe.
Complete step by step answer:
We know that iron (II, III) oxide is the chemical compound with formula Fe3O4. It occurs in nature as the mineral magnetite. It is one of a number of iron oxides, the others being iron(II) oxide (FeO), which is rare, and iron(III) oxide Fe2O3 also known as hematite.
Fe3O4 is known as ferrosoferric oxide is an iron oxide, Iron (II,III) oxide (Fe3O4).
In this compound three types of bonding of Fe are there. In which one Fe is bonded with two oxygens and other each of two Fe are bonded with three oxygen.
ForFe3O4, two Fe atoms have an oxidation state of +3 and one of +2. Structure ofFe3O4:
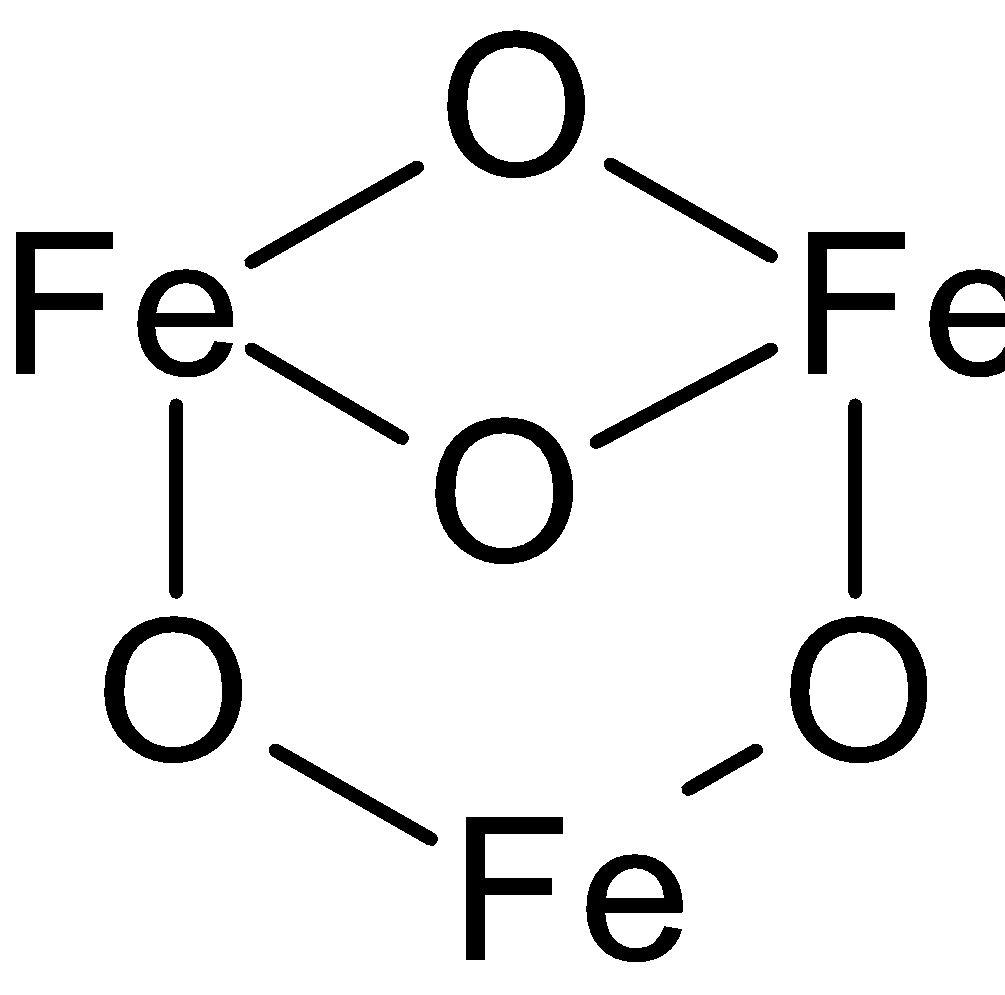
Here we take the average of oxidation state:
Let the oxidation state of Fe is ‘n’
So, 3n -8 = 0
‘n’ = +8/3
So, the correct answer is “Option B”.
Note: Instead of averaging the oxidation states, we must keep them separated. This is called fragmenting, which occurs if there is an ionic compound, and the ions can be separated.
