Question
Question: The oxidation potential (in volt) of a hydrogen electrode at pH = 7 and $p_{H_2}$ = 1 atm is $\left...
The oxidation potential (in volt) of a hydrogen electrode at pH = 7 and pH2 = 1 atm is
(Given 2.303FRT=0.06)
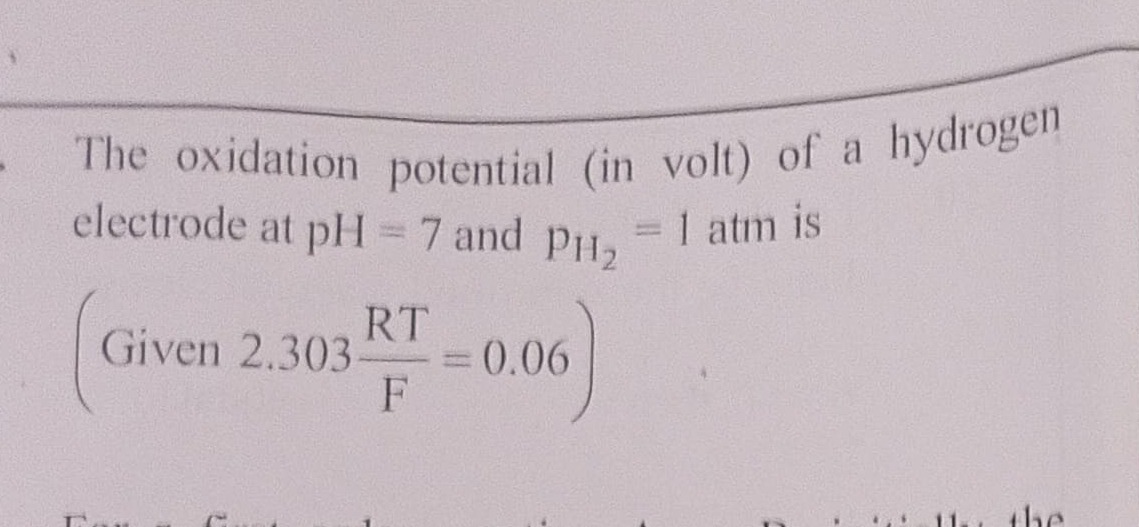
0.42
Solution
To find the oxidation potential of a hydrogen electrode, we first write the oxidation half-reaction and then apply the Nernst equation.
1. Oxidation Half-Reaction: The oxidation of hydrogen gas to hydrogen ions is represented as: 21H2(g)⇌H+(aq)+e− For this reaction, the number of electrons transferred, n=1. The standard oxidation potential for a hydrogen electrode, Eox0, is 0 V.
2. Nernst Equation: The Nernst equation for the oxidation potential (Eox) is: Eox=Eox0−nF2.303RTlog[Reactant][Product] Substituting the species from the half-reaction: Eox=Eox0−nF2.303RTlogpH21/2[H+]
3. Given Values:
- Eox0=0 V
- n=1
- 2.303FRT=0.06 (given)
- pH = 7
- pH2=1 atm
4. Calculate [H+] from pH: pH = −log[H+] 7=−log[H+] log[H+]=−7 [H+]=10−7 M
5. Substitute values into the Nernst Equation: Eox=0−10.06log(1)1/210−7 Eox=−0.06log(10−7) Using the property log(ab)=blog(a): Eox=−0.06×(−7)log(10) Since log(10)=1: Eox=−0.06×(−7) Eox=0.42 V
The oxidation potential of the hydrogen electrode at pH = 7 and pH2 = 1 atm is 0.42 V.
