Question
Question: The oxidation numbers of the sulphur atoms in peroxymonosulfuric acid \[({H_2}S{O_5})\]and peroxodis...
The oxidation numbers of the sulphur atoms in peroxymonosulfuric acid (H2SO5)and peroxodisulfuric acid(H2S2O8) are respectively
A.+8 and +7
B.+3 and +3
C.+6 and +6
D.+4 and +6
Solution
We should be aware of the fact that the maximum oxidation state for sulphur in a compound is +6.
In general, we observe that oxo-acids of sulphur have +4 and +6 oxidation state. Since sulphur does not exhibit negative oxidation state in oxo acids, we can eliminate such options if mentioned in the question.
Formula used:
To solve such questions with higher accuracy, we should be well-versed with the structures of the compound to be dealt with.
Oxidation number is calculated on the basis of structure.
Complete step by step answer:
Before knowing the term oxidation number, we should be familiar with what oxidation and reduction means.
So, we will study it in the tabular form.
Reduction
Gain of hydrogen (electropositive element).
Loss of oxygen (electronegative element).
Gain of electrons.
Oxidation
Loss of hydrogen (electropositive element).
Gain of oxygen (electronegative element).
Loss of electrons.
An atom either gains or loses electrons in order to form a chemical bond with another atom.
This positive or negative value shows the effective charge of a particle (be it an atom or element) is called oxidation number.
Peroxomonosulphuric acid, commonly called Caro's acid, has the molecular formulaH2SO5. It contains one peroxy linkage in which the oxidation state of oxygen is -1 instead of -2. The diagram is as shown below:
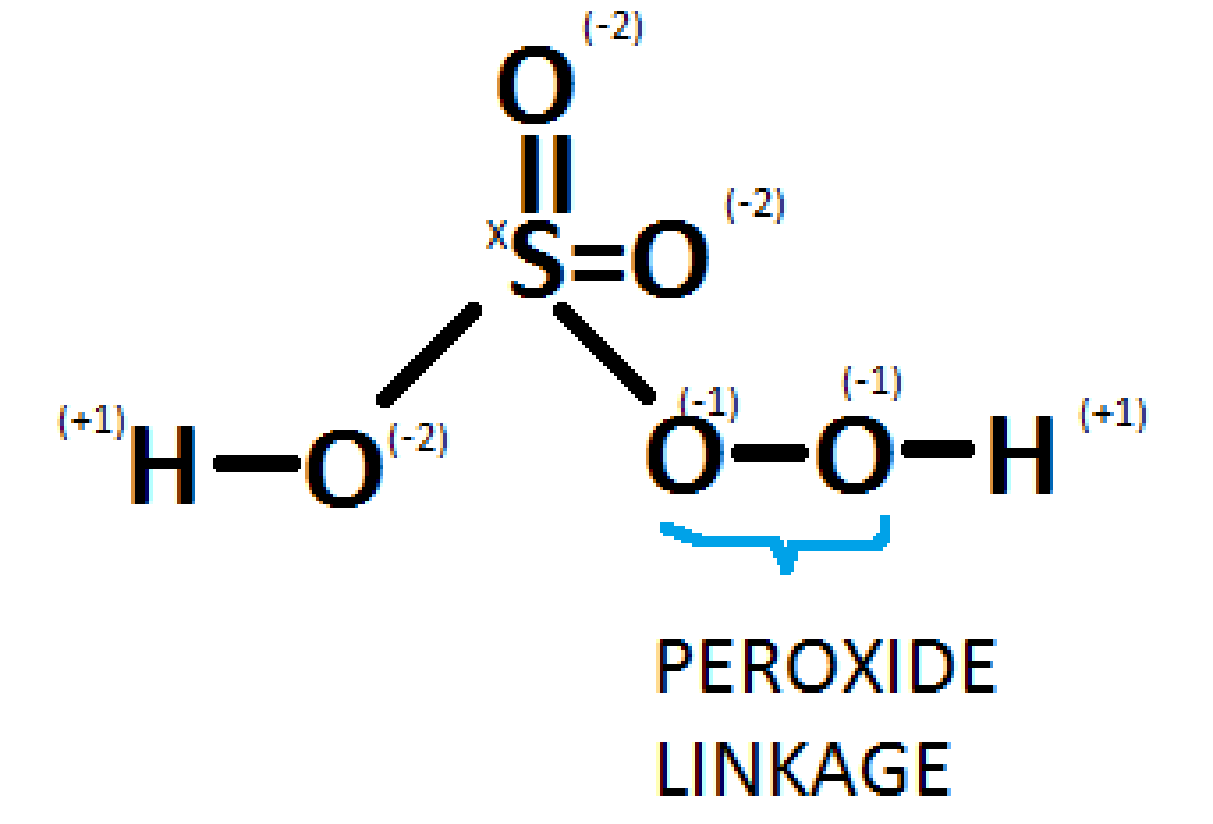
Solving the question for x,
We get
Thus, the oxidation state for peroxymonosulfuric acid(H2SO5) is +6.
Now, we will look into a detailed discussion for peroxodisulfuric acid(H2S2O8), also known as Marshall’s acid.
As we see a peroxy linkage in peroxymonosulfuric acid(H2SO5) , similarly when we draw the structure forH2S2O8, we get one peroxy linkage in which the oxidation state of oxygen is – 1 , rather than -2. Following is the structure forH2S2O8
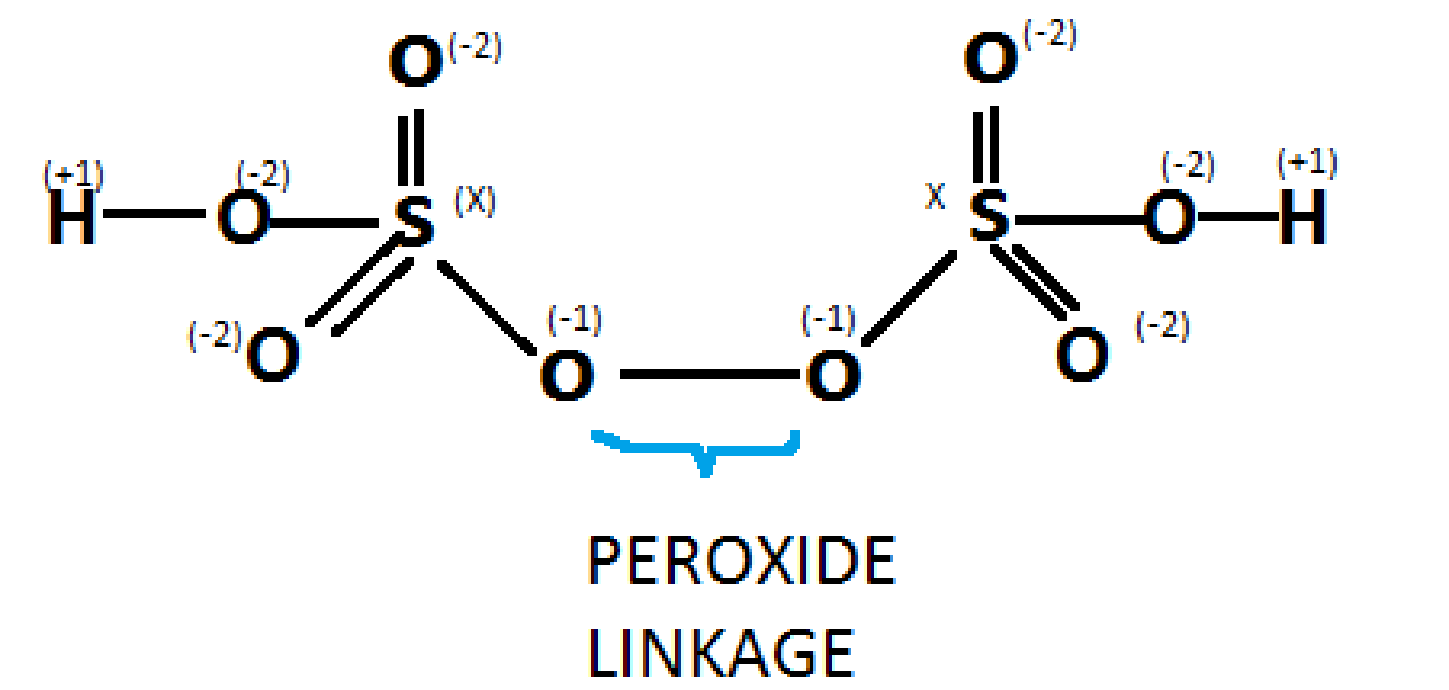
As we can see in the diagram,
We have to find a value of x in order to get the oxidation state of sulphur in H2S2O8
Solving for x,
We deduce that:
Therefore, C. option is the correct option for the given question.
Additional Information:
Oxo-acids of sulphur are sp3hybridized.
H2S2O8 is a powerful oxidizing agent used in the industry.
H2SO5 is widely used in hydrometallurgy industries.
Note:
The oxidation number of a free element is zero.
An Interesting fact to note about the sulphur;
Cyclo−undecasulphurmonoxide,S11O, has the lowest oxidation number for sulphur.
