Question
Question: The oxidation number of sulfur in \({ H }_{ 2 }{ S }_{ 2 }{ O }_{ 8 }\) is: A) \({ +2 }\) B) \({...
The oxidation number of sulfur in H2S2O8 is:
A) +2
B) +4
C) +6
D) +7
Solution
Hint: An oxidation number is a number assigned to an element in a chemical combination which represents the number of electrons lost (or gained, if the number is negative), by an atom of that element in the compound.
Complete step-by-step answer:
The structure of H2S2O8 is
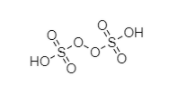
Let, the oxidation number of sulfur is = x
In peroxydisulfuric acid, two oxygen atoms are involved in peroxide linkage, so the oxidation number will be −1 each. The remaining oxygen atoms have as usual a −2 charge.
So, the oxidation number of sulfur in H2S2O8 can be calculated as:
2+2x+2(−1)+6(−2)=0
⇒2+2x−2−12=0
⇒2x−12=0
⇒2x=12
⇒x=12/2
⇒x=+6
Hence, the oxidation number of sulfur in H2S2O8 is +6.
The correct option is C.
Additional Information:
There are some rules for assigning oxidation numbers to an atom: The rules have been formulated on the basis of the assumption that electrons in a covalent bond belong entirely to the more electronegative atom.
Oxidation number (O.N) of:
atoms in free elemental state (like H2, Na, O2, Ag etc) = 0
Oxidation number of simple monatomic ions = Charge on them ( For example : Halogens (like Fluorine, chlorine) = -1, Na+= +1, Ca+2= +2 etc)
Oxygen = -2; in peroxides(-1); F2O (+2); F2O2 (+1)
Hydrogen = +1; however in metal hydrides it is (-1)
Sum of O.N. of all the atoms in molecules = 0
Sum of O.N. of atoms in polyatomic ions = overall charge on them
Note: The possibility to make a mistake is that you may choose option D. As in this compound, peroxide is present and the oxidation number of these are −1, not −2, so instead of 8 oxides, there are 6 oxides and 2 peroxides.
