Question
Question: The oxidation number of \(Cr\) in \(Cr{{O}_{5}}\). A. +10 B. +8 C. +6 D. +4...
The oxidation number of Cr in CrO5.
A. +10
B. +8
C. +6
D. +4
Solution
ry to recall that oxidation number of an element is the charge which an atom of the element has in its ion or appears to have when present in combined state with another atom. Now, by using this you can easily find the correct option from the given ones.
Complete step by step answer:
- We know that the net charge found on a neutral compound is always 0.
- So, taking the whole compound of CrO5 its oxidation number is zero (since it is neutral).
- The structure of CrO5 is given below:
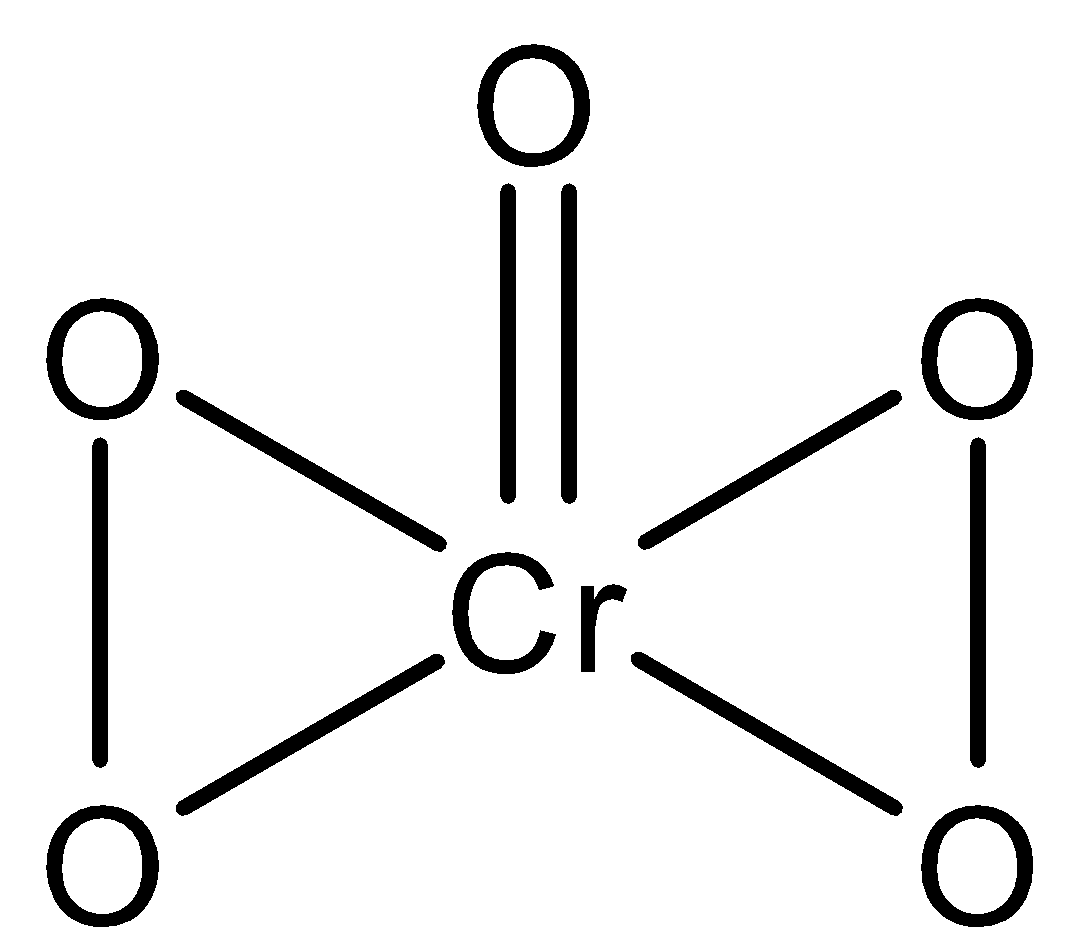
- In the above butterfly structure of CrO5, one Cr atom is attached to four oxygen atoms by single bond and double bonded with one oxygen atom. Also, there are two peroxide linkages (−O−O− ).
- By looking at the above structure we observe that four oxygen atoms are involved in peroxide linkages. So, the oxidation state of each of the four oxygen atoms involved in peroxide linkage is taken as -1 and the one which is double bonded with chromium is normal so its oxidation state is -2.
- Since, we want to know the oxidation number of Cr, so we consider the oxidation number of Cr(chromium) to be x.
- Calculation:
