Question
Question: The organic compound obtained during the addition of HBr to propene in the presence of peroxide cata...
The organic compound obtained during the addition of HBr to propene in the presence of peroxide catalyst is-
[A] 2-bromopropane
[B] 2-bromopropene
[C] 1-bromopropane
[D] 1-bromopropene
Solution
Due to the presence of peroxide, the product formed will be Anti-Markovnikov as bromide will undergo peroxide effect. As a result, bromine will be added to the less substituted carbon.
Complete answer:
For the conversion of alkenes to alkanes or alkynes to alkenes by the addition of hydrogen halide, the product formed is either Markovnikov product or Anti-Markovnikov based on whether it is formed by following the rule or opposing the rule.
According to Markovnikov's Rule, during the addition of an unsymmetrical reagent to an unsymmetrical alkene, the positive part of the reagent attaches to that carbon which leads to a more stable carbocation.
But in the presence of peroxide, the product formed is anti-markovnikov , due to the peroxide effect.
Here, HBr is added to propene, there is a possibility of formation of two carbocation formation.
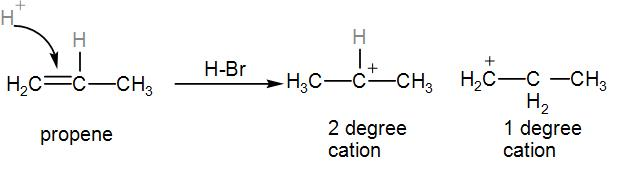
One is 2∘, as it has 2 other substitutes while the other is 1∘ has only 1 carbon substitute. The 2∘ carbocation is more stable.
According to markovnikov, the more stable carbocation should be formed.
But here, due to peroxide effect, the 1∘ carbocation is formed, which will give us the following product-
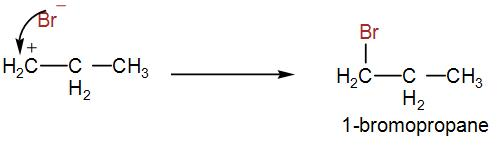
As bromine is added to the first carbon therefore, 1-bromopropane is formed.
Therefore, the correct answer is option [C] 1-bromopropane.
Note:
Here, if peroxide was absent, the product formed would be different. Bromine would be added to the stable carbocation, which is the 2∘carbon here and the product thus formed would be 2-bromopropane.
Alkene is converted to alkane in presence of HBr and the reaction is addition therefore the options with propene are incorrect.
