Question
Question: The order of relative stability of the contributing structure is: ...
The order of relative stability of the contributing structure is:
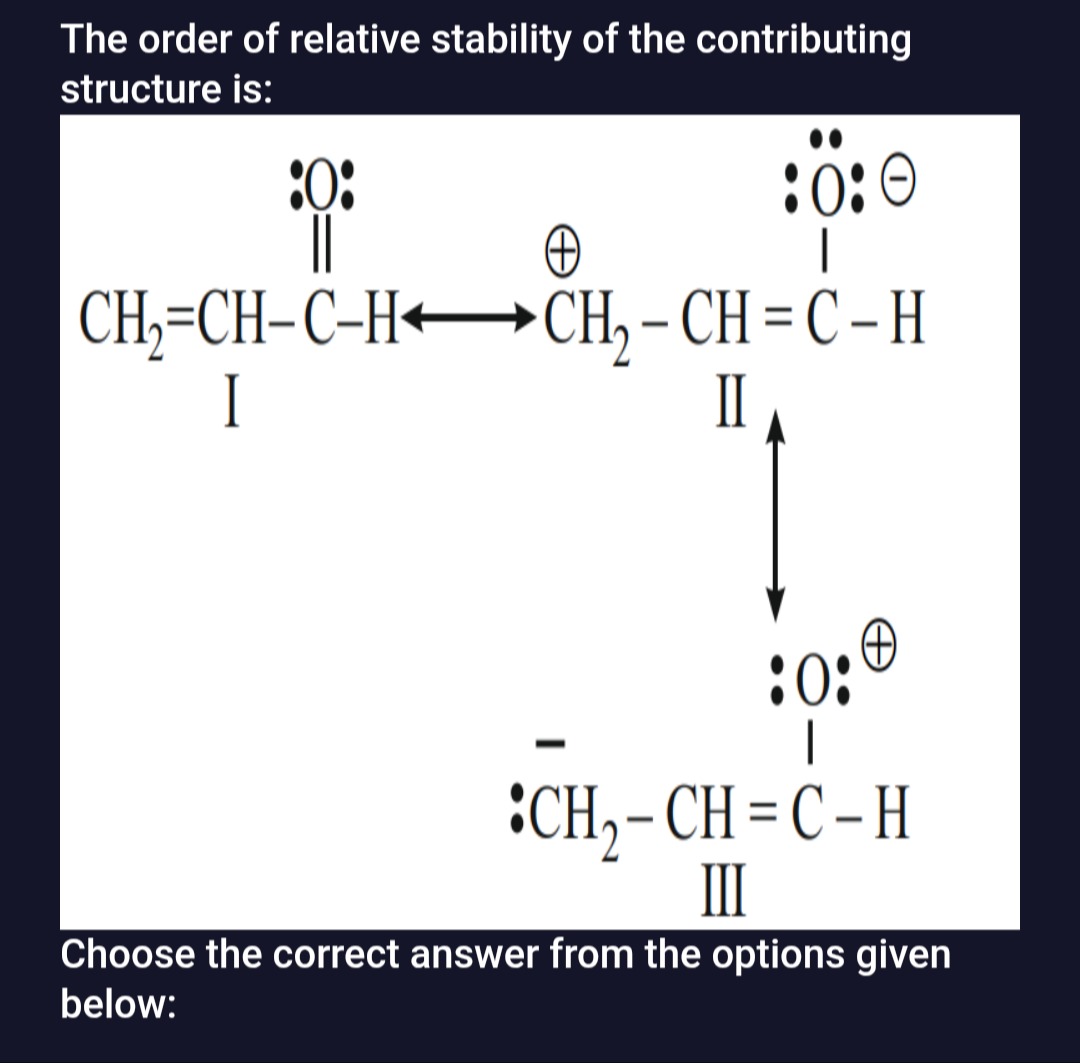
A
I > III > II
B
I > II > III
C
II > I > III
D
III > II > I
Answer
I > III > II
Explanation
Solution
The stability of resonance structures is determined by the following factors, prioritized as follows:
- Neutral structures are more stable than charged structures.
- Structures with complete octets for all atoms are more stable.
- Structures with more covalent bonds are more stable.
- Structures with negative charges on more electronegative atoms and positive charges on less electronegative atoms are more stable.
Analyzing the given structures:
- Structure I: CH2=CH−O⋅⋅−H is neutral and all atoms have complete octets. This makes it the most stable.
- Structure II: CH2⊕−CH=O⋅⋅−H has a positive charge on carbon (C1) and a negative charge on oxygen. Crucially, C1 has an incomplete octet (6 electrons).
- Structure III: :−CH2−CH=O⊕−H has a negative charge on carbon and a positive charge on oxygen. All atoms have complete octets.
Comparing the structures:
- Structure I is the most stable as it is neutral with complete octets.
- Structure III is more stable than Structure II because all atoms have complete octets, even though there is a charge separation with a positive charge on the more electronegative oxygen.
- Structure II is the least stable due to the presence of an incomplete octet on the carbon atom (C1).
Therefore, the order of stability from most stable to least stable is I > III > II.
