Question
Question: The order of reaction A → Product can be given by the expression(s) [where r = rate of reaction, $[A...
The order of reaction A → Product can be given by the expression(s) [where r = rate of reaction, [A]1 and [A]2 are concentration at t1 and t2 respectively]
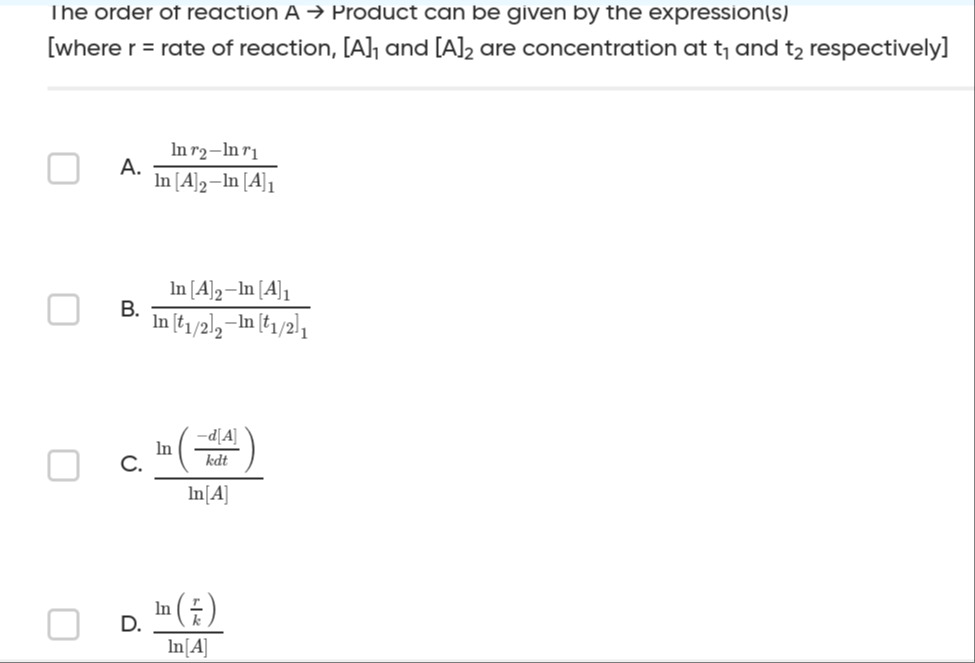
A
ln[A]2−ln[A]1lnr2−lnr1
B
ln[t1/2]2−ln[t1/2]1ln[A]2−ln[A]1
C
ln[A]ln(kdt−d[A])
D
ln[A]ln(kr)
Answer
A, C, D
Explanation
Solution
The rate law for a reaction A → Product is r=k[A]n.
- Option A: Taking natural log of the rate law, lnr=lnk+nln[A]. For two data points (r1,[A]1) and (r2,[A]2), subtracting the equations lnr2−lnr1=n(ln[A]2−ln[A]1) yields n=ln[A]2−ln[A]1lnr2−lnr1.
- Option B: The half-life for n=1 is t1/2∝[A]01−n. Taking natural log, lnt1/2=C′+(1−n)ln[A]0. For two data points, ln[t1/2]2−ln[t1/2]1=(1−n)(ln[A]2−ln[A]1). This leads to ln[t1/2]2−ln[t1/2]1ln[A]2−ln[A]1=1−n1, not 'n'.
- Option C and D: From the rate law r=k[A]n, divide by k to get kr=[A]n. Taking natural log, ln(kr)=nln[A]. Thus, n=ln[A]ln(kr). Option C uses r=−d[A]/dt, so kdt−d[A]=kr, making C equivalent to D.
