Question
Question: The order of basic strength of the given basic nitrogen atoms is : ...
The order of basic strength of the given basic nitrogen atoms is :
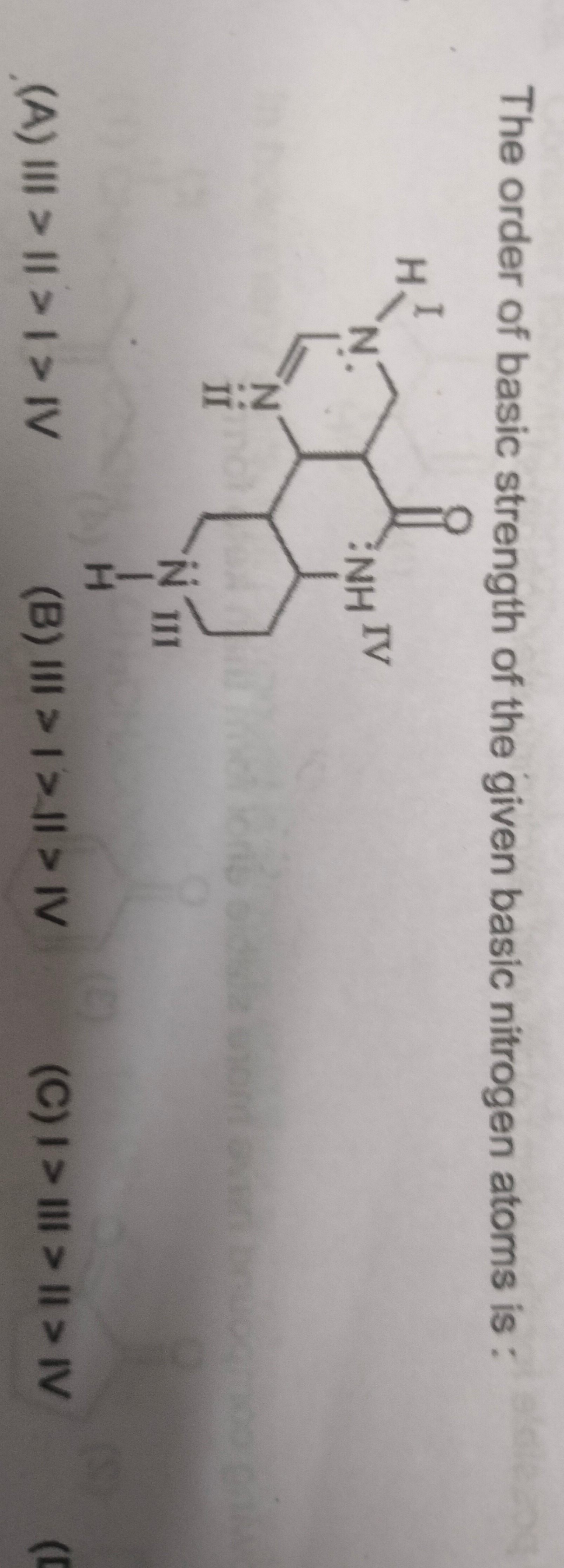
III > II > I > IV
III > I > II > IV
I > II > II > IV
B
Solution
The basic strength of a nitrogen atom depends on the availability of its lone pair of electrons for donation. Factors affecting basicity include:
- Hybridization: Lower s-character (sp3 > sp2 > sp) leads to higher basicity because the lone pair is held less tightly by the nucleus.
- Resonance: If the lone pair is involved in resonance (delocalization), its availability for donation decreases, thus reducing basicity.
- Inductive Effects: Electron-donating groups (+I) increase electron density on nitrogen, enhancing basicity. Electron-withdrawing groups (-I) decrease electron density, reducing basicity.
Let's analyze each nitrogen atom:
-
Nitrogen (IV) - Amide Nitrogen: The nitrogen atom labeled IV is part of an amide group (-NH-C=O). Its lone pair is extensively delocalized into the adjacent carbonyl group through resonance. This resonance stabilization makes the lone pair highly unavailable for protonation, rendering N(IV) the weakest base among all.
-
Nitrogen (III) - Secondary Aliphatic Amine: The nitrogen atom labeled III is a secondary amine in a saturated six-membered ring. It is sp3 hybridized. Its lone pair is localized and not involved in any resonance. Aliphatic amines are generally strong bases because the sp3 orbital has less s-character, and the alkyl groups are electron-donating, increasing electron density on the nitrogen. Thus, N(III) is the strongest base.
-
Nitrogen (I) - Imine Nitrogen: The nitrogen atom labeled I is an imine nitrogen (part of a C=N double bond). It is sp2 hybridized. Its lone pair is in an sp2 orbital and is not involved in the aromaticity of the fused ring system (it points outward from the ring). It is available for donation.
-
Nitrogen (II) - Pyridine-like Nitrogen: The nitrogen atom labeled II is a pyridine-like nitrogen within the aromatic pyrimidine ring. It is also sp2 hybridized, and its lone pair is in an sp2 orbital, not contributing to aromaticity, and is available for donation. However, N(II) is directly adjacent to the strong electron-withdrawing carbonyl group (C=O). The inductive effect (-I) of the carbonyl group pulls electron density away from N(II), significantly reducing its basicity compared to N(I).
Comparing N(I) and N(II): Both are sp2 hybridized. N(II) is adjacent to an electron-withdrawing carbonyl group, which makes it less basic than N(I), which is not directly adjacent to such a strong electron-withdrawing group. Therefore, N(I) > N(II).
Overall Order: Based on the analysis:
- N(III) is the strongest (sp3, localized, no strong EWG).
- N(I) is stronger than N(II) (sp2, less affected by EWG than N(II)).
- N(II) is weaker than N(I) (sp2, strong -I effect from C=O).
- N(IV) is the weakest (resonance delocalization into C=O).
Thus, the order of basic strength is: III > I > II > IV.
