Question
Question: The order of basic strength of \[{C_6}{H_5}N{H_2}(1),\,{C_2}{H_5}N{H_2}(2),{({C_2}{H_5})_2}NH(3)\] a...
The order of basic strength of C6H5NH2(1),C2H5NH2(2),(C2H5)2NH(3) and NH3(4) is:
A. 1<4<2<3
B. 1<2<3<4
C. 4<3<2<1
D. 3<2<4<1
Solution
We know that basicity of amines can be determined with the help of some factors such as steric factors, hydrogen bonding and electronegativity. Using these factors, we can find out the correct option because the basicity of amines is due to the lone pair of electrons on nitrogen atoms.
Complete step-by-step answer:
We already know that amines are basic in nature because they have a lone pair of electrons present on nitrogen atoms. They have a strong tendency to donate those lone pairs of electrons to electron acceptors.
The basicity of amines basically depends on the electronic properties of the substituents such as alkyl groups enhance the basicity whereas aryl groups diminishes it. Also, it depends on the degree of salvation of the protonated amine i.e., steric hindrance by the groups present on nitrogen.
Now comes the important point that the aliphatic amines are more basic as compared to aromatic amines. The reason behind this is the lone pair present on nitrogen atoms is delocalised in case of aromatic amines. So, the availability of the lone pair of electrons will be less and thus will be less basic than aliphatic amines.
Looking at these points, let us arrange the given compounds in the increasing order of basic strength. We have aniline, the only aromatic compound in all the four compounds given as the rest are aliphatic in nature. And since we know aromatic amines are least basic, so aniline will be least basic out of all.
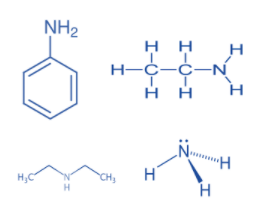
Ethyl amine and diethyl amine are more basic than ammonia due to the presence of electron releasing ethyl groups which have +I effect on the amino group. This increases electron density on nitrogen due to which the lone pair of electrons on nitrogen atoms can be easily donated.
Hence the correct option is (A).
Note: Alkyl groups always tend to donate electrons to the more electronegative nitrogen atom. The inductive effect is responsible for the electron density on the alkylamine's nitrogen which is greater than the nitrogen of ammonia. Due to this reason, primary, secondary, and tertiary alkyl amines are more basic than ammonia.
