Question
Question: The orbital picture of singlet carbene \(\left( {:C{H_2}} \right)\) can be drawn as: (A).  can be drawn as:
(A).
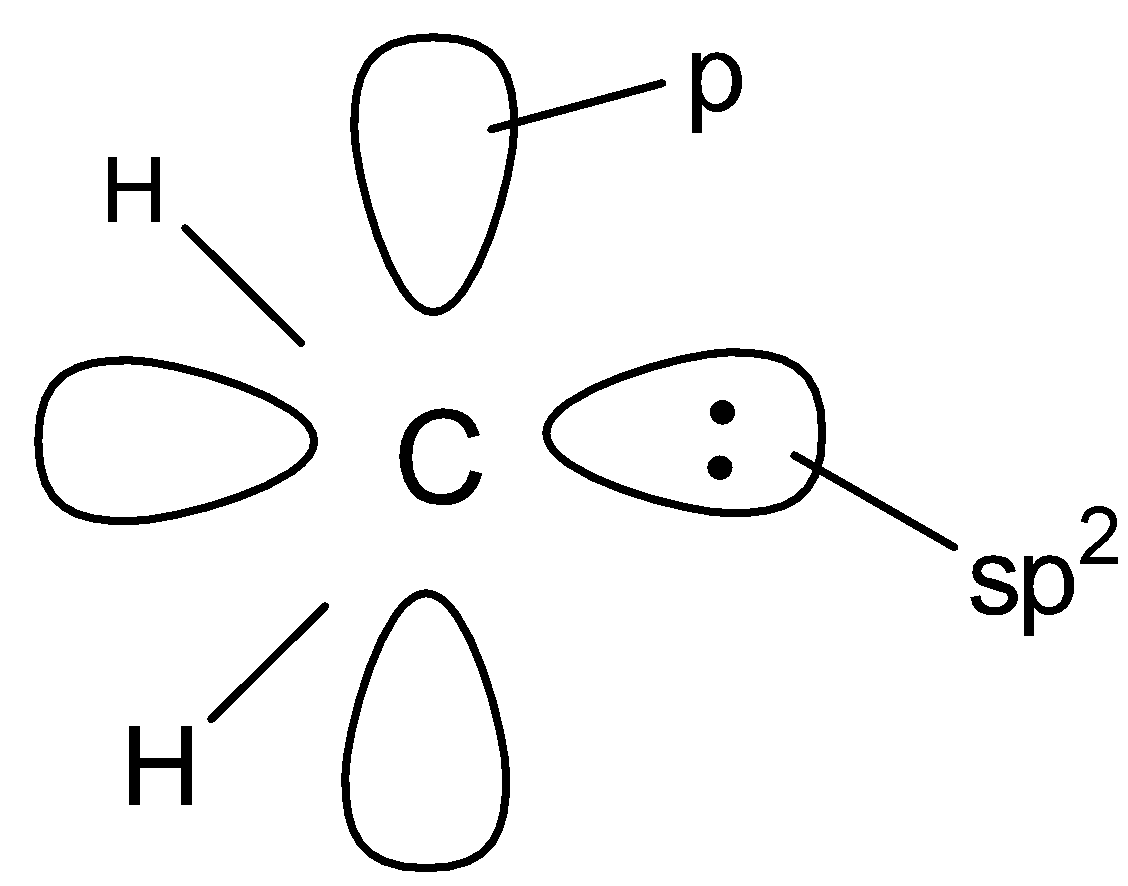
(B).
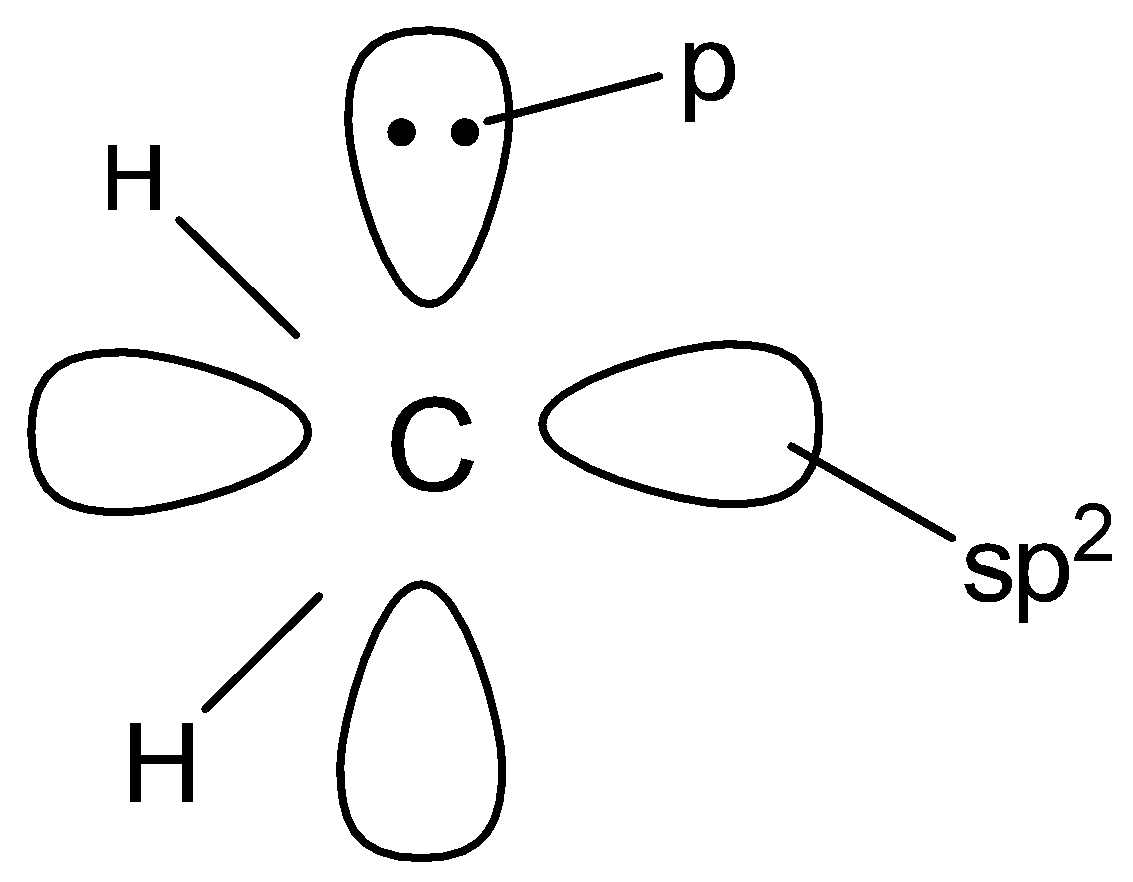
(C).
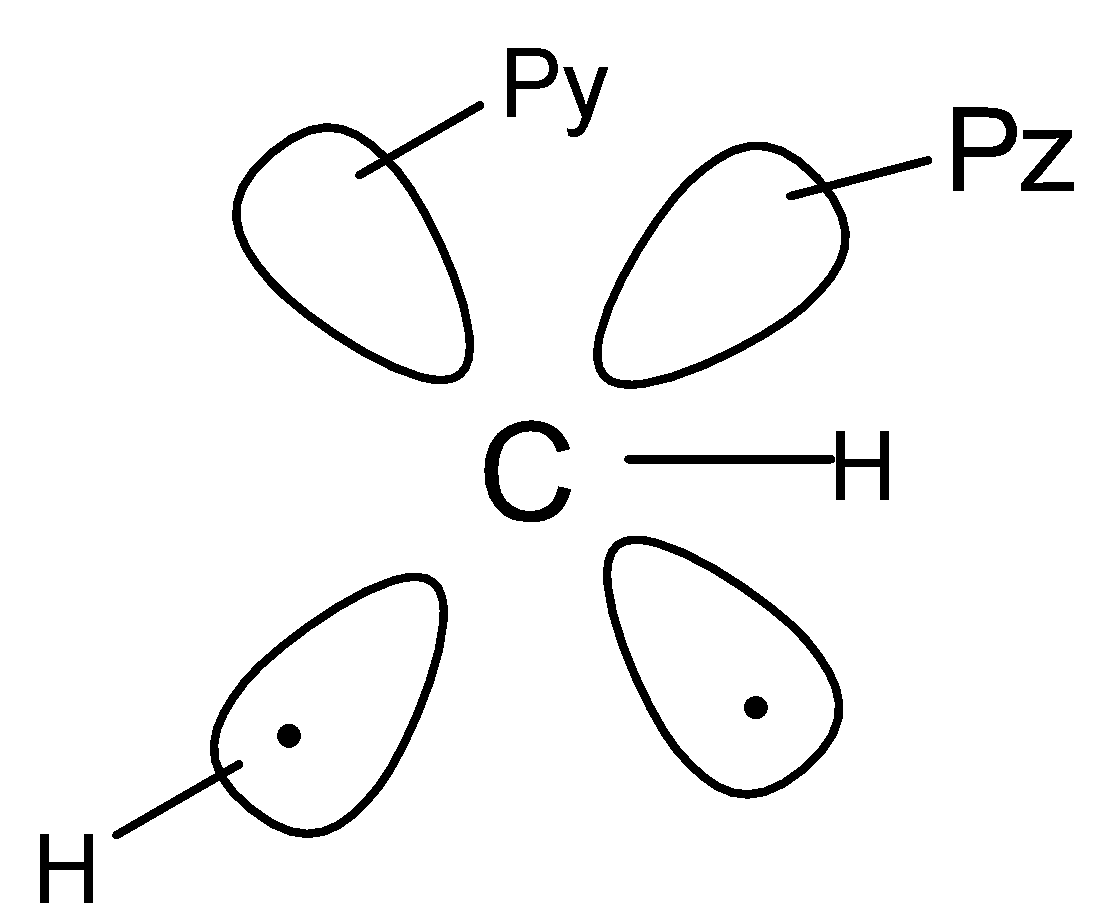
(D). None of these.
Solution
Neutral divalent carbon species in which the carbon atom is bonded to two monovalent atoms or groups and also contains two non- bonding electrons are called carbenes .
Complete answer:
Carbenes are generally produced either by photolysis ( irradiation with UV light ) or thermolysis or pyrolysis ( action of heat ) of diazoalkanes or ketenes .
Now talking about the orbital structure of carbene , there are two types of carbenes , that is singlet carbene and triplet carbene . Singlet carbenes are less stable than triplet carbenes since triplet carbenes have a linear structure and behave as a diradical whereas singlet carbenes have bent structure .
In singlet carbenes , the central carbon atom is sp2 hybridised . Two of the sp2 - hybridised orbitals form two σ - bonds with two monovalent atoms or groups while the third sp2 - hybridised orbital contains two non - bonding electrons . The unhybridized p - orbital is , however, empty . Thus , a singlet carbene has a bent structure .
So according to the above description , the orbital structure of carbene will be
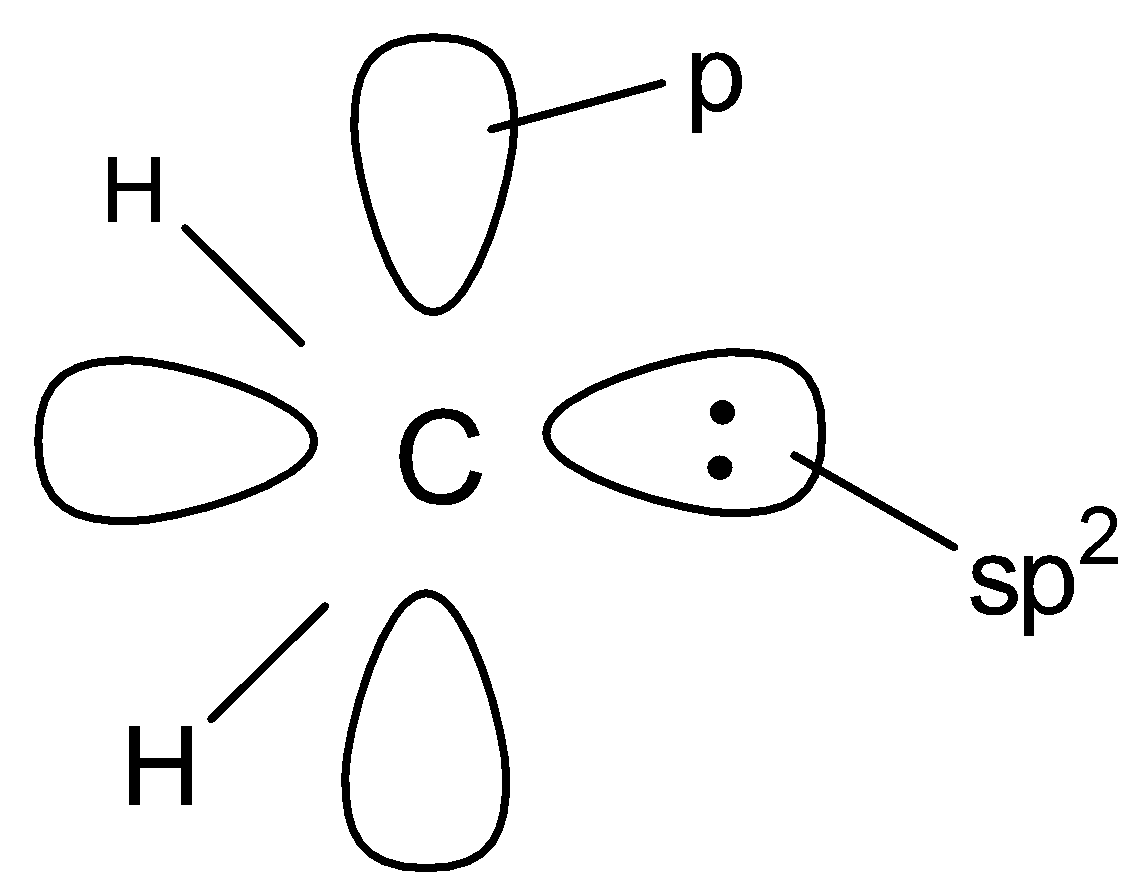
Hence option A is correct .
Note:
Just like carbocations , carbenes are short lived highly reactive chemical species since the central carbon atom has only six electrons in its valence shell and thus has a strong tendency to complete its octet by gaining two more electrons . Carbenes , thus behave as Lewis acids or electrophiles .
