Question
Question: The orbital angular momentum of an electron (\(l = 1\)) makes an angle of \({\text{4}}{{\text{5}}^{\...
The orbital angular momentum of an electron (l=1) makes an angle of 45ofrom Z axis. The Lz of electron will be
(LZ = m2Πh)
A.2×2Πh
B.0×2Πh
C.32Πh
D.3×2Πh
Solution
When the Schrodinger equation is solved for the hydrogen atom ,it is found that there are 3 characteristic quantum numbers n,l,m which represent an orbital in the hydrogen atom. n is the principal quantum number , l is the orbital/azimuthal quantum number, m is the magnetic quantum number.
For an atom containing more than one electron we need a fourth quantum number called spin quantum number represented by s, it is used to distinguish between two electrons in an orbital.
Complete answer:
When we look at the 4 quantum numbers
n is the principal quantum number which represents the principal energy level of an otherwise shell in which the electron revolves around the nucleus. It can have any integral value other than zero, n=1,2,3,4...........
l is the orbital quantum number also known as azimuthal quantum number which determines the subshell to which the electron belongs . For a n value lcan have values 0→n−1.
m is the magnetic quantum number representing the orientation of atomic orbital in space , which depend on the values of l, so it can have values (2l+1)
s is the spin quantum number which represent spin of electron its value is ±21
Now we can learn about angular momentum. Angular momentum is a physical quantity which plays an important role in understanding the electronic structure of an atom. Here we can consider the hydrogen atom as an example in which the electron in the hydrogen atom moves in a circular orbit with constant speed / angular velocity.
So any moving object with mass , possess momentum . so angular momentum deals with rotating or spinning objects.
Angular momentum can be calculated by using formula
L=Iω where I is the moment of inertia and ωis the angular velocity
For a particle of mass m moving in a circle of radius r
I=mr2 , ω=rv where v is the velocity of moving particles.
L=mvr
And the orbital angular momentum of a single electron is given by the equation
L=l(l+1)2Πh where l is the orbital angular momentum quantum number.
z component of orbital angular momentum is given by the equation
LZ=m2Πh where m is the magnetic moment quantum number.
Complete step by step solution: it has clearly written as l=1 so here the electron is in p subshell. Here movement of an electron in an orbital makes an angle of 45o from the LZaxis in the xyplane.
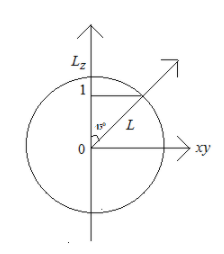
Orbital angular momentum is given by the equation L=l(l+1)2Πh
Here l=1 ,then we get L=1(1+1)2Πh=22Πh
Here we have to calculate orbital angular momentum in LZaxis
From the above figure Cosθ=LLZ
So we get LZ=Lcosθ,where θ=450 hence the value of Cosθ=2
∴LZ=22Πh×2=2Πh
So, here the correct answer is option C .
Note:
It has to be noted that each electron in an atom has a unique set of quantum numbers , that is according to Pauli's exclusion principle no two electrons in an atom can have the same set of four quantum numbers.
