Question
Question: The optically active molecule is: A)  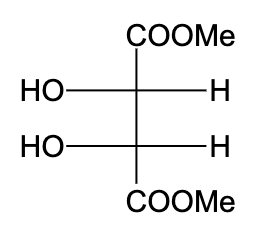
B) 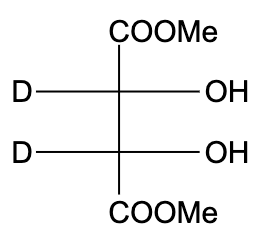
C) 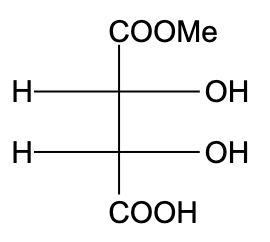
D) 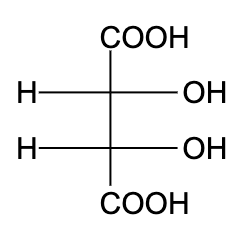
Solution
Optical isomers are called enantiomers. They have identical chemical as well as physical properties. The Optical isomerism is associated with a plane of symmetry of the compound.
Complete step by step answer:
- Molecules which are optical isomers are known as enantiomers. They have identical chemical and physical properties. Only their effect on the plane of polarised light and reaction with the other chiral molecules are not the same.
- A chiral molecule that rotates through a plane of polarised light is said to be optically active. If the molecule rotates in a clockwise direction, we call that rotation a dextrorotatory rotation. However, if the light rotates counter-clockwise, we call that rotation as levorotatory.
- To identify the optically active molecule given in the question, we will consider the plane of symmetry of the compound. If there is an element of the plane of symmetry, the compound is optically inactive, if not found, and then it is optically active.
-In option (A), the compound is not optically active due to the presence of a plane of symmetry. 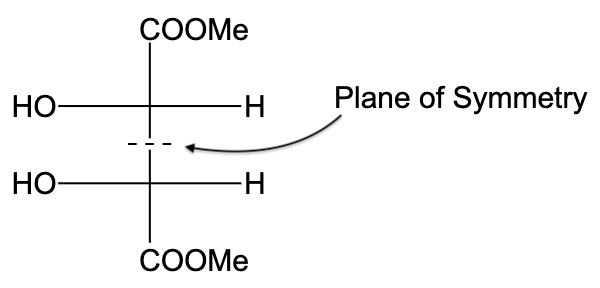
- In option (B), the compound is not optically active due to the presence of a plane of symmetry 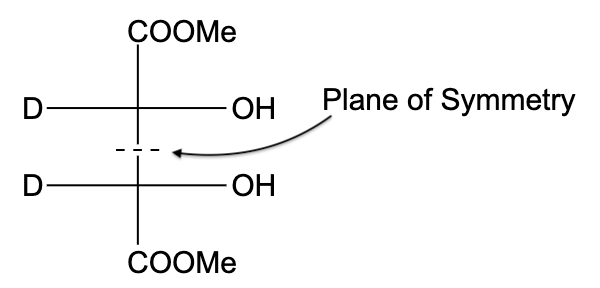
- In option (C), the compound is optically active, due to the presence of a plane of symmetry. 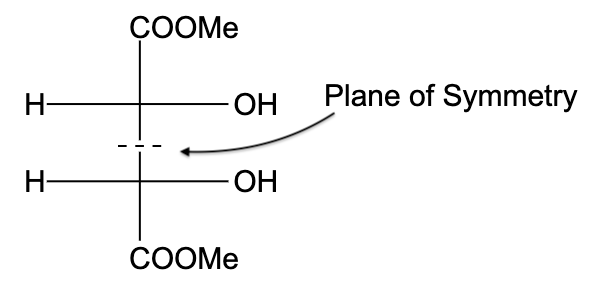
- In option (D), the compound is not optically active due to the presence of a plane of symmetry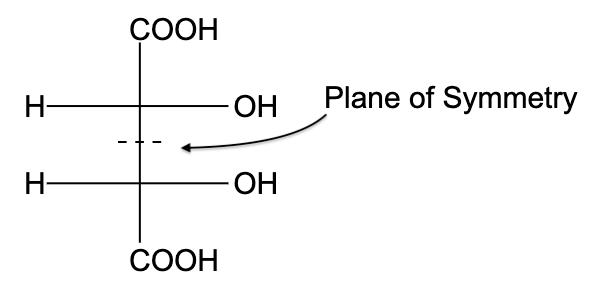
So, the correct answer is “Option C”.
Note: Chiral molecules are optically active. Therefore, when a beam of plane-polarized light passes through a chiral molecule, it interacts with the molecule in such a way that the angle of the plane of oscillation rotates.
