Question
Question: The numbers of lone pair(s) on \({\text{Xe}}\) in \({\text{Xe}}{{\text{F}}_{\text{2}}}\) and \({\tex...
The numbers of lone pair(s) on Xe in XeF2 and XeF4 are, respectively:
A) 2 and 3
B) 4 and 1
C) 3 and 2
D) 4 and 2
Solution
Using the periodic table calculate the total number of valence electrons of XeF2 and XeF4. Draw the Lewis dot structures of both the compounds and determine the number of lone pair on Xe in XeF2 and XeF4.
Formula Used: Number of valence electrons of an element = Group number of an element
Number of valence electrons of an element = Sum of the valence electrons of all atoms
Complete step by step answer:
Calculate the total number of valence electrons of XeF2 and XeF4.
Number of valence electrons of an element = Group number of an element
An element Xe is a Noble gas element and present in group number 8 of the periodic table. While F is halogen and present in group number 7 of the periodic table.
Number of valence electrons of Xe = 8
Number of valence electrons of F = 7
{\text{Total no}}{\text{. of valence electrons of Xe}}{{\text{F}}_{\text{2}}} = {\text{ (No}}{\text{. of Xe atoms) (No}}{\text{. of valence electrons of Xe) + (No}}{\text{. of F atoms) }} \\\
{\text{ (No}}{\text{. of valence electrons of F)}} \\\
Total no. of valence electrons of XeF2= (1) (8) + (2)(7) = 22 electrons
Similarly, we can calculate the total number of valence electrons of XeF4 as follows:
Total no. of valence electrons of XeF4= (1) (8) + (4)(7) = 36 electrons
Using the total number of valence electrons draw the Lewis dot structures of XeF2 and XeF4 as follows:
A less electronegative atom is always a central atom except for H atom.
So, in both the compounds the central atom is Xe.
Skeleton structures of XeF2 and XeF4 are
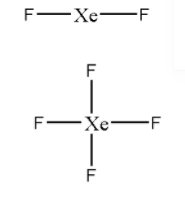
Now, let us distribute the valence electrons to complete the octet of surrounding atoms.
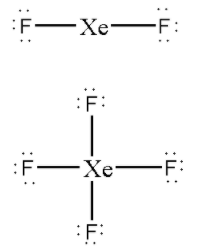
Now, let us distribute the remaining valence electrons around the central atom.
Out of total 22 valence electrons of XeF2 we have distributed 4 electrons in bonding and 12 electrons as lone pairs around F atoms.
So, now distribute the remaining 6 electrons as 3 lone pairs on the central Xe atom.
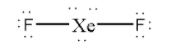
Thus, there are 3 lone pairs on the central Xe atom in XeF2.
Out of total 36 valence electrons of XeF4 we have distributed 8 electrons in bonding and 24 electrons as lone pairs around F atoms.
Now, distribute the remaining 4 electrons as 2 lone pairs on the central Xe atom.
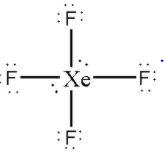
Thus, there are 2 lone pairs on the central Xe atom inXeF4.
Hence, the correct option is (C) 3 and 2.
Note: Here in both the compounds central Xe atom show an exception to the octet rule. In the case of XeF2 a central Xe atom is surrounded by 10 electrons and in the case of XeF4 a central Xe atom is surrounded by 12 electrons.
