Question
Question: The numbers of isomeric amines possible for the formula \[{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{...
The numbers of isomeric amines possible for the formula C3H9N.
A) 4
B) 3
C) 5
D) 6
Solution
Isomers are the compounds having the same molecular formula but different structural formulae. There are different types of isomers like position isomers, chain isomers, functional group isomers, stereoisomers, etc.Even they have the same chemical formula they show different physical and chemical formulae and hence, we can differentiate the isomers.
Complete answer:
Here, the chemical formula of the amine is given as C3H9N. Amine is one of the functional groups in which a nitrogen atom is bonded to three other atoms and possesses one lone pair of electrons on it.
Here, it is important to note that nitrogen can form three bonds, carbon can form a maximum of four bonds and hydrogen can form a maximum of one bond.
The possible isomers for the amine C3H9Nare given as follows:
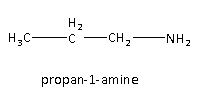
Propan-1-amine is the first isomer of the amine given which is primary amine as one of the hydrogen atoms is replaced by the alkyl group.
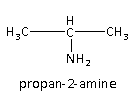
Propan-2-amine is the second possible isomer of the given amine. It is also primary amine as one of the hydrogen atoms is replaced by the alkyl group.
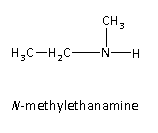
N-methylethanamine is the third possible isomer of the given amine. It is secondary amine because two hydrogen atoms are replaced by the alkyl groups.
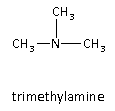
Trimethylamine is the fourth possible isomer of the given compound. Here, all hydrogen atoms are replaced by the alkyl groups therefore, it is a tertiary amine.
Thus, four possible structures can be drawn using the given chemical formula of the amine.
Here, option(B) 3, is the incorrect answer to the question.
Here, option(C) 5, is also incorrect.
Now, option(D) 6, is also an incorrect answer.
Hence,the correct option is (A).
Note: Amines are derivatives of ammonia hence, based on the numbers of the hydrogen atoms replaced by the carbon atoms of the alkyl or aryl groups amines are classified as primary amines, secondary amines, and tertiary amines.In the case of the primary amines,one hydrogen atom is replaced by the carbon of the alkyl or aryl group.In the case of the secondary amines,two hydrogen atoms are replaced by the carbon of the alkyl or aryl group.In case of the tertiary amines,all three-hydrogen atom is replaced by the carbon of the alkyl or aryl group.
