Question
Question: The number of water molecules directly bonded to the metal centre in \(CuS{{O}_{4}}.5{{H}_{2}}O\) is...
The number of water molecules directly bonded to the metal centre in CuSO4.5H2O is ………………..
Solution
In CuSO4.5H2O the metal centre is Cu (Copper). Here the metal ion is six coordinated. The coordination group surrounding the cupric ion is 4 molecules of water and two oxygen atoms of sulphate ions and the fifth water molecule is held between the water molecules attached with the cation and oxygen atoms that are attached with sulphate ions.
Complete answer:
Copper (II) sulphate which is also known as copper sulphate is the inorganic compounds having a chemical formula of CuSO4.(H2O)x where the range of x can be 0 to 5 but the most common form is of pentahydrate having a chemical formula of CuSO4.5H2O.
Cupric sulphate is a salt which is formed when copper oxide is treated with sulfuric acid and forms a large bright blue crystal having five molecules of water in it. It dissolves in water exothermically and give the aqua complex of [Cu(H2O)6]2+ having octahedral molecular geometry.
In commercial copper sulphate the percentage of copper is 98% with very small amount of water but in its blue hydrous form (CuSO4.5H2O) the percentage of copper is 25.47%, 38.47% of sulphate and the water by mass percentage is 36.06%.
In the question we have asked to calculate the water molecules that are directly bonded with CuSO4.5H2O. Here the metal ion is six coordinated. The coordination group surrounding the cupric ion is 4 molecules of water and two oxygen atoms of sulphate ions and the fifth water molecule is held between the water molecules attached with the cation and oxygen atoms that are attached with sulphate ions.
Fifth water molecules form hydrogen bonding and are not coordinated and are deeply embedded in the crystal. So only four water molecules form a coordinated bond and are directly attached with the metal centre and the fifth bond only form a hydrogen bond.
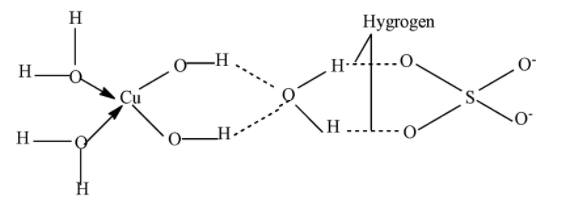
Hence the number of water molecules directly bonded with metal centre is 4
Note: The pentahydrate CuSO4.5H2O is also known as blue vitriol. It is used for agriculture purposes like pesticides, feed additives, etc. and it is insoluble in alcohol. To produce the anhydrous salt the hydrate is heated to 150⁰ C. Sometimes it is also used as an electrolyte for electroplating baths and batteries and for medicinal purposes like fungicide, astringent, etc.
