Question
Question: The number of unpaired electrons in the \(F{e^{3 + }}\) ion (atomic no. =\(26\) ) is A) \(5\) B)...
The number of unpaired electrons in the Fe3+ ion (atomic no. =26 ) is
A) 5
B) 6
C) 2
D) 8
Solution
We need to know that the symbol of Iron atoms is Fe. The atomic number of the iron is 26 . The electron filling in every energy level of the atom forms from lower energy level to higher energy level. The electron filling in one orbital, first it fills in a single electron then filling wise it pairs it that orbitals. The atom loses that electron in its orbital to form cation. The number of electrons losing is equal to the charge of the cation forming after the loss of the electron.
Complete step by step answer:
As we know that the symbol of Iron atom is Fe and it forms cation by loss of electron. Here, iron loses three electrons to form Fe+3 . The atomic number of Fe is 26 and electronic configuration is [Ar]4s23d6 . The electrons filled in iron is given as,
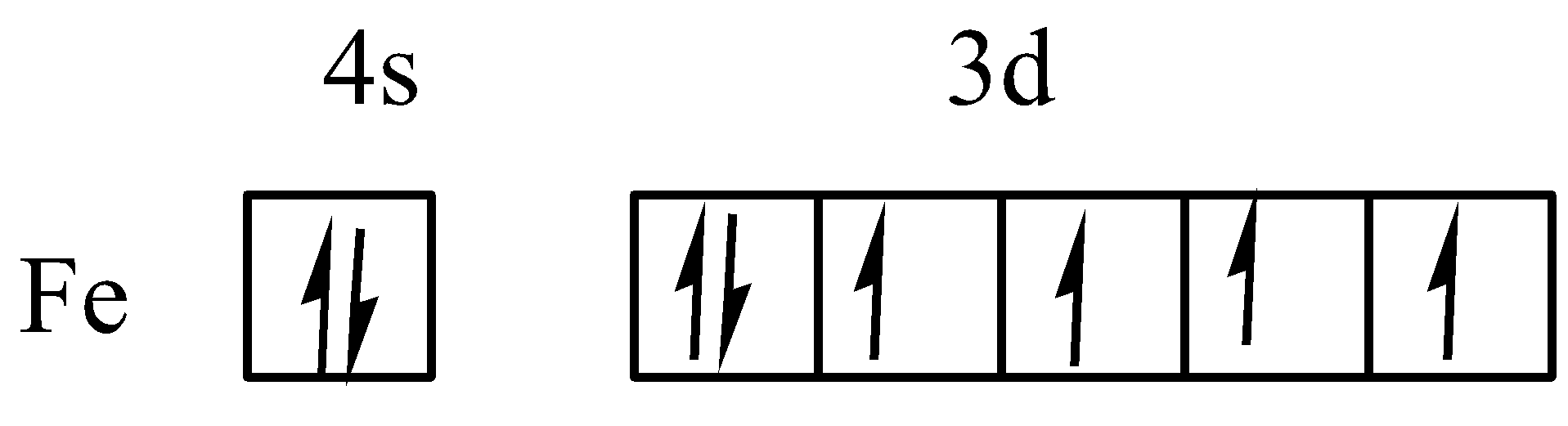
The three electrons are losing from the iron atom. First two electrons are loss from 4S2 , the electronic configuration of iron change to [Ar]3d6 and the symbol of iron cation is Fe+2 .
In electronic configuration of Fe+2 is [Ar]3d6 . In 3d6 , one paired electron and four unpaired electrons in d orbital. That third electron is removed from paired one and forms Fe+3 and electronic configuration is [Ar]3d5 . There are five unpaired electrons in Fe+3 in d orbital.
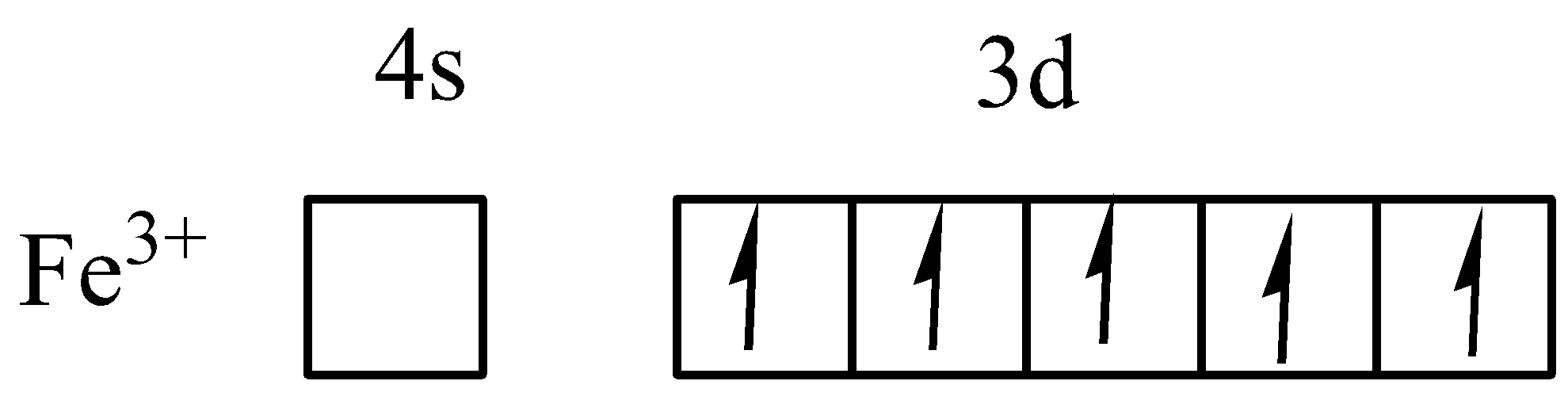
Therefore, the option A is correct, because Fe+3 have 5 unpaired electrons.
Note:
We need to remember that the iron is d block element. The d block elements are also called transition elements, because they have various oxidation states in elements. In atoms, electrons are filling and losing on the basis of Hund’s rule, Pauli’s exclusion principle and Aufbau principle. Half-filled and completely filled orbitals are more stable than partially filled orbitals. That is the reason Fe+3 is more stable than Fe. In Fe+3 is half-filled orbitals but Fe is partial filled in d orbitals.
