Question
Question: The number of unpaired electrons in Ferrous Ion is: A. \(2\) B. \(3\) C. \(4\) D. \(5\)...
The number of unpaired electrons in Ferrous Ion is:
A. 2
B. 3
C. 4
D. 5
Solution
The nucleus of the atom contains the protons and the neutrons. The outermost regions of the atom are called electron shells and contain the electrons which are negatively charged. Electrons revolve in different orbits having different energy levels. For arrangement of electrons in each shell, Bohr bury rule is applied.
Complete step by step solution:
Ferrous ion has the atomic number 26 and the electronic configuration is: 1s22s22p63s23p64s23d6
Each atomic orbital of an electron has capacity to contain two electrons which are of opposite spin value i.e., if one electron is in the state of clockwise then another will be in anticlockwise state and this was explained by Pauli exclusion principle. Always make sure to remember the concepts of pairing and that is going to help you in understanding the concept of diamagnetic and paramagnetic nature of a substance that will be further useful
We will fill up the corresponding orbitals with electrons until you find out the electron arrangement, any left with one is called unpaired electron. The box of the outer electronic configuration is given as:
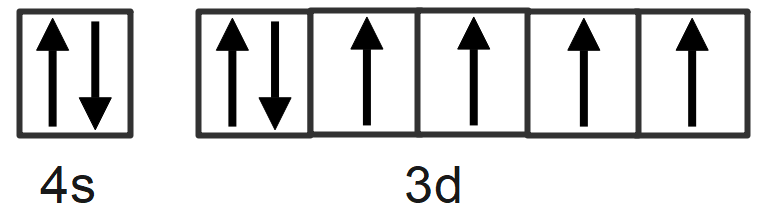
Therefore, the number of unpaired electrons in ferrous ion is 4.
So, the correct answer is Option C
Note: Whenever two electrons are paired together in an orbital, or their total spin is zero, they are diamagnetic electrons. Atoms with all diamagnetic electrons are called diamagnetic atoms. A paramagnetic electron is an unpaired electron. An atom is considered paramagnetic if even one orbital has a net spin.
